已传文件:photo/1600935908.png
[According to "Biosensors and Bioelectronics" October 2015 report] Title: Simultaneous detection of H5 and H9 influenza A virus antigens based on quantum dot immunoassay technology (author Feng Wu, etc.)
Influenza (influenza for short) is an acute respiratory infectious disease caused by influenza virus, which is mainly spread by air droplets. There are four types of influenza viruses: A, B, C, and D. Among them, the influenza epidemic caused by A is the most widespread and serious, and the B often causes small outbreaks. Influenza is extremely harmful and has a wide host range. It is a highly transmissible infectious disease and zoonotic disease. It is a major epidemic that international organizations such as WHO, FDA, and OIE jointly advocate for global joint prevention and control. Influenza has a rapid onset. Although most of them are self-limited, they can develop into severe influenza due to complications such as pneumonia. A few severe cases progress rapidly, which can be due to acute respiratory distress syndrome (ARDS) and/or multiple organ failure And death. Severe influenza mainly occurs in high-risk groups such as the elderly, young children, pregnant women or those with chronic underlying diseases, and can also occur in the general population. It is estimated that there are 3 to 5 million cases of severe influenza worldwide each year, causing 250,000 to 500,000 deaths. my country is a country with a high incidence of influenza and a key international surveillance. According to the survey of seasonal influenza diseases in my country, the annual excess mortality caused by influenza in the north and south of China is 18.0 per 100,000 and 11.3 per 100,000 respectively, of which deaths are caused by respiration and circulation. System diseases accounted for 49.2% and 46.2%.
Influenza has a huge impact on our people’s livelihood and health every year, and it also causes economic losses of up to billions of yuan for our country. In order to strengthen the prevention and treatment of influenza, reduce the occurrence of severe influenza, and reduce the mortality rate, the country issued the "Influenza Clinical guidelines such as the “Diagnosis and Treatment Plan (2019 Edition)” and the “Consensus of Chinese Adult Influenza Diagnosis and Treatment Standards and Emergency Department Experts” regulate the clinical diagnosis and treatment of influenza. The guidelines clarify that the emergency influenza emergency screening process should be initiated as soon as possible for suspected cases of emergency influenza. It is recommended that the influenza paper method be used in the emergency department to quickly screen for suspected cases. At the same time, the guidelines also clearly point out that the clinical diagnosis of influenza should be combined with epidemiological history, clinical manifestations and etiological examinations: clinical influenza-like cases (high fever accompanied by cough or sore throat) should be promptly tested for influenza virus antigens. Patients who are positive should be isolated in time and given antiviral treatment depending on their condition. The result of rapid antigen detection of influenza virus plays a decisive role and is the basis for subsequent clinical treatment.
However, the current clinical knowledge of rapid influenza antigen detection technology is that false negative results are more common, especially in the influenza season. A negative result cannot rule out a suspected influenza diagnosis in patients, such as a negative rapid clinical antigen test but highly suspected influenza Or severe cases need to be re-examined with more accurate viral nucleic acid testing to clarify the condition. The main reason for this problem is that rapid detection reagents for influenza virus antigens based on colloidal gold technology are commonly used in clinical practice. This technology has the problem of low sensitivity, and often misses detection during influenza outbreak seasons, and the clinical diagnosis accuracy rate is low, which cannot meet the requirements. Clinical needs. Therefore, rapid antigen detection reagents with high accuracy are urgently needed clinically to meet the increasing demand.
In recent years, the application of quantum dot materials in the field of medical testing has gradually increased. It has the advantages of fast detection speed and high detection accuracy. It is mainly used in the field of in vitro diagnostic reagents, especially in the field of point-of-care testing (POCT) for disease markers and pathogens. The diagnosis of antigen is widely used. Due to the quantum size effect, fluorescent nanocrystals of different sizes can produce light of multiple colors when irradiated by light of a single wavelength. As the size increases, the peak position of the light emission will gradually red shift. According to the light-emitting range of the fluorescent nanomaterial itself, its excitation light source can adopt a variety of light sources, which avoids the problem of high cost caused by traditional luminescent materials that must be detected by laser optical instruments. In addition, the fluorescent nanocrystalline labeling material overcomes the problems of traditional luminescent materials, such as easy decomposition, short luminescence time, wide emission line, high background, and low detection sensitivity. It has stronger luminescence, better monochromaticity, and is suitable for photobleaching. It has the advantage of better stability, and its detection sensitivity is also greatly increased. Therefore, the use of fluorescent quantum dots as the labeling material can realize ultra-micro detection of biomolecules.
At present, the technique of using quantum dot fluorescent microsphere labeling technology and applying the principle of membrane chromatography double antibody sandwich method to detect influenza A and (or) B influenza virus antigens in samples has been widely used in clinical practice. The principle is to drop the processed sample into the sample holes corresponding to the two detection areas of the test card A and B. When influenza A and (or) B viruses are present in the sample, the virus antigens and fluorescent microspheres are labeled After the combination of influenza A and (or) B influenza virus nucleoprotein monoclonal antibody I, when chromatographed to the detection line, it will be captured by the coated influenza A and (or) B influenza virus nucleoprotein monoclonal antibody II. The detection line positions are gathered to form fluorescent strips A line and/or B line. Under ultraviolet irradiation with a wavelength of 365nm, the result can be determined by naked eyes to be positive for influenza A and/or B viruses, or through the fluorescent layer The test paper interpreter determines that the result is positive for influenza A and (or) B influenza virus. On the contrary, when there are no influenza A and B viruses in the sample or the virus titer is below the minimum detection limit, no fluorescent band appears on the test line, and the result is negative at this time. Regardless of whether the sample contains influenza A or B virus, a fluorescent band C line will appear at the position of the quality control line in the two detection areas A and B. The quality control line shows that the fluorescent band is the standard for judging whether the chromatography process is normal. It is also used as the internal control standard of the test card (Figure 1).
Figure 1 | Principles of rapid influenza antigen detection technology based on quantum dot fluorescence immunochromatography

For pathogen detection of influenza virus, virus culture and nucleic acid detection (common PCR technique) are currently used clinically as the gold standard for detection. In particular, the PCR method has high sensitivity and accuracy, and it is currently a common detection technique for the diagnosis of influenza virus infection in laboratories. . In order to evaluate the detection effects of fluorescence immunochromatography and colloidal gold technology in clinical application, clinical samples were tested by fluorescence immunochromatography and colloidal gold technology respectively, and the PCR detection results were compared, and the detection sensitivity of the two detection technologies were compared. , Specificity and other technical indicators to clarify the difference in detection performance of the two detection technologies. The detection results are shown in Table 1.
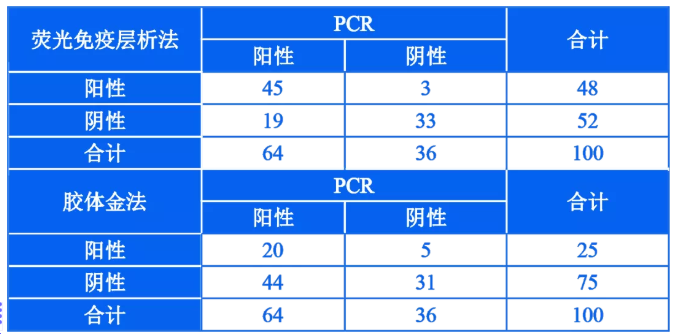
Table 1 |Comparison of clinical sample test results and PCR test results by fluorescence immunochromatography and colloidal gold technology
Analyze the above detection results, and obtain the performance comparison of the two detection technologies in Table 2.
Table 2 | Comparison of clinical sample detection performance between fluorescence immunochromatography and colloidal gold technology
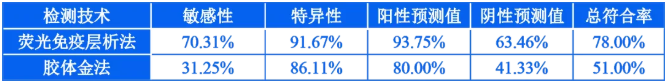
From the above results, it can be seen that the PCR method is the gold standard, and the indicators of the fluorescence immunoassay technique are higher than the colloidal gold technique, and the total coincidence rate has increased from 51% to 78%. It can be seen that the fluorescence immunochromatography technology based on quantum dot technology has obvious advantages over colloidal gold technology in the accuracy and effectiveness of the detection of influenza virus antigens. It can increase the clinical diagnosis accuracy rate to more than 70%, and has better clinical The application value is expected to solve the problem of low accuracy of the original colloidal gold method. It is expected that the wide application of this technology in clinical practice can bring good results for clinical diagnosis in the current large-scale influenza outbreak, ensure the effective diversion and treatment of influenza patients, and ultimately achieve the purpose of effectively controlling the spread of influenza epidemic. It is currently urgently needed clinical testing. technology.
Source of information: Shenzhen Yilifang Biotechnology Co., Ltd.
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately







