已传文件:photo/1602727149.png
This article was written by the corresponding author-Professor Liu Junfeng, and the first author-Qin Yongji, PhD student who was invited to write. We sincerely thank them for their academic support!
1. Research background:
Metal organic framework materials (MOFs) have received extensive attention in the field of catalysis due to their high specific surface area and adjustable structure. However, most of the existing MOFs are microporous channels, which are not conducive to the diffusion of guest molecules and restrict the catalytic efficiency of MOF-based catalysts. In recent years, Professor Liu Junfeng’s research group has carried out research work on the structure regulation and diffusion optimization of MOF-based catalyst materials, and proposed a "shorten substrate diffusion path strategy" to improve the catalytic efficiency of MOFs and made a series of progress (Nat. Commun. 2017) , 8, 14429; Angew. Chem. Int. Ed. 2017, 56, 5512; Nano Res. 2017, 10, 3826). However, how to reduce the mass transfer resistance of MOFs microporous channels to the substrate while shortening the mass transfer path, and to explore the relationship between the channel structure-diffusion efficiency-catalytic performance from the micro-scale still remains a huge challenge.
2. Introduction to the article:
In response to the above problems, Professor Liu Junfeng and Professor Xu Haijun from the State Key Laboratory of Chemical Resources Effective Utilization of Beijing University of Chemical Technology and Associate Professor Liu Wenxian from Zhejiang University of Technology proposed the use of different metals and ligand coordination stability differences and crystal burst nucleation to prepare hollow cores -Method of mesoporous structure MOFs materials. Related results were published on ACS Catalysis. This work used Cr/Al bimetal MIL-101 (S-MIL) as the precursor, and prepared hollow-mesoporous MIL-101 (HM-MIL) through a simple selective etching process. Diffusion experiments and molecular dynamics simulations proved that the hollow-mesoporous structure can effectively improve the efficiency of substrate diffusion. The results of the catalytic oxidation of p-chlorostyrene and benzyl alcohol showed that HM-MIL showed excellent performance when used directly as a catalyst and as a catalyst carrier.
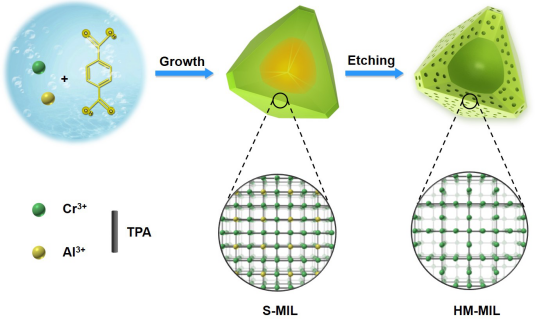
Figure 1. Schematic diagram of HM-MIL synthesis.
3. Article content:
1. Preparation and structure characterization of HM-MIL
The solid Cr/Al bimetal MIL-101 (S-MIL) was prepared by hydrothermal reaction, and then hollow-mesoporous MIL-101 (HM-MIL) was prepared by selective etching (Figure 1). The electron micrographs confirmed the solid structure of S-MIL and the hollow-mesoporous structure of HM-MIL (Figure 2a-b). The high-angle annular dark-field image and the element surface scan photo show the uniform distribution of Cr/Al element in the MOF crystal, which proves the successful doping of Al element (Figure 2c-d). The EDX results show that Al element is significantly reduced after selective etching, which proves that Al element is unstable and preferentially etched (Figure 2e). DFT pore size analysis shows that the microporous structure is partially transformed into a mesoporous structure during the selective etching process (Figure 2f).

Figure 2. a) S-MIL scan and transmission electron microscope photos; b) HM-MIL transmission electron microscope photos; c) S-MIL high-angle ring dark field image and EDX surface scan photos; d) HM-MIL high-angle ring Dark field image and EDX surface scan photo; e) XRD pattern; f) DFT aperture distribution.
2. Research on the diffusion of HM-MIL
Using p-chlorostyrene as a probe, the diffusion properties of MOFs with solid, hollow and hollow-mesoporous structures were studied (Figure 3a). The results of the adsorption diffusion experiment and the desorption diffusion experiment show that the hollow-mesoporous HM-MIL has the fastest diffusion rate (Figure 3b-c). In addition, the use of molecular dynamics simulation to calculate the mean square displacement of the molecule also proved that the construction of mesopores can improve the diffusion efficiency (Figure 3d).
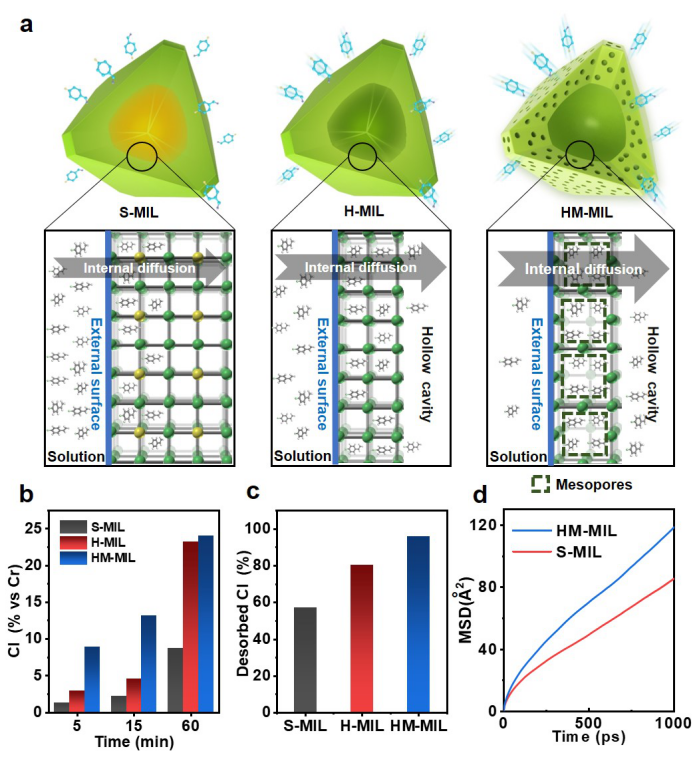
Figure 3. a) Schematic diagram of diffusion rate; b) adsorption experiment; c) desorption experiment; d) molecular dynamics simulation molecular mean square displacement graph.
3. Study on the oxidation performance of p-chlorostyrene
The catalytic oxidation results of p-chlorostyrene show that the performance of HM-MIL is better than that of S-MIL and H-MIL, which is attributed to the mesoporous structure/defects caused during the etching process (Figure 4a-b). The catalytic activity of HM-MIL did not decrease significantly after three cycles of use, indicating that it has good cycle stability (Figure 4c).
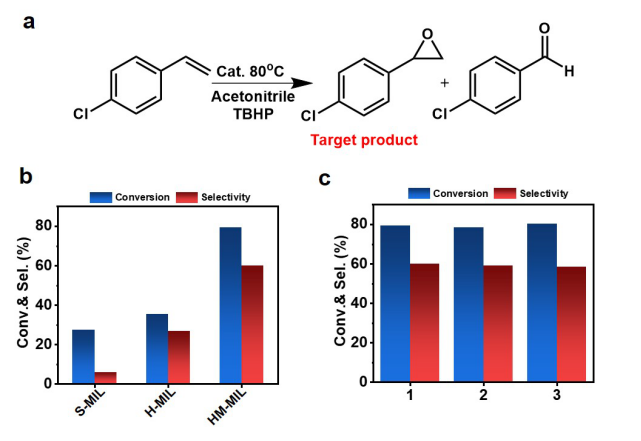
Figure 4. a) Schematic diagram of p-chlorostyrene oxidation; b) catalytic performance of samples with different structures; c) stability test.
4. Study on the oxidation of benzyl alcohol catalyzed by HM-MIL supported Pd
Furthermore, the author used HM-MIL as the carrier of Pd nanoparticles and determined the structural advantage of hollow-mesoporous structure as the catalyst carrier. Transmission electron micrographs and XRD results confirmed the uniform loading of Pd nanoparticles and the structure maintenance of MOF carrier (Figure 5a-c). The results of catalytic oxidation of benzyl alcohol showed that Pd/HM-MIL has good catalytic activity and stability (Figure 5d-f).

Figure 5. Pd/HM-MIL photos of a) TEM and b) high-resolution electron microscopy; c) XRD pattern; d) schematic diagram of benzyl alcohol oxidation; e) catalytic performance of different samples; f) stability test
4. Summary and outlook:
In this paper, using the difference in coordination stability between different metals and ligands and the burst nucleation of crystals, hollow-mesoporous MOFs were prepared by selective etching process. Combining molecular dynamics simulations and diffusion/catalysis experiments proved that the hollow-mesoporous structure improves substrate diffusion and catalytic efficiency. This work not only helps to understand the intrinsic relationship between MOF pore structure-substrate diffusion-catalytic efficiency from the molecular scale, but also provides new ideas for the design of porous catalysts with complex pore structures.
Five, thanks:
Thanks to the key research and development program of the Ministry of Science and Technology (2018YFA0702000), the National Natural Science Foundation of China, the Beijing Natural Science Foundation of China (2204089), the special funds for basic scientific research operations of central universities, and the high-performance computing platform of Beijing University of Chemical Technology.
Click "Read Original" at the end of the article to go directly to the document.
Authors: Yongji Qin, Xu Han, Yaping Li, Aijuan Han, Wenxian Liu*, Haijun Xu*, and Junfeng Liu*
Title: Hollow Mesoporous Metal-Organic Frameworks with Enhanced Diffusion for Highly Efficient Catalysis
Published in: ACS Catalysis, doi: 10.1021/acscatal.0c01432.








