

Highlights of this article]
1. The dual function of CTAB: Intercalation treatment of Ti3C2 MXene by CTAB can effectively open the interlayer of MXene and provide an opportunity for the in-situ growth of Na3Ti5O12 nanowires between MXene layers. In addition, CTAB can stabilize the interface of Na3Ti5O12 nanowires grown in situ on MXene, making the nanowires more uniform in size and longer.
2. The 1D/2D Na3Ti5O12/MXene hybrid nanostructure derived from MXene exhibits excellent electrochemical performance as a metal Na anode base material due to its abundant Na nucleation sites and interpenetrating nanopores.

Sodium ion batteries have shown great potential in large-scale energy storage equipment because of their high energy density, low raw materials and abundant resources. Metal sodium has become the most ideal negative electrode material because of its low reduction potential and relatively high specific capacity. At the same time, for emerging sodium battery systems (such as sodium-sulfur, sodium-air and sodium-carbon dioxide batteries, etc.), metallic sodium is considered to be the only anode material that can be paired with these high-performance cathode materials. The theoretical energy of these high-energy batteries It can reach 3-4 times of lithium ion or sodium ion batteries.
Despite the above advantages, due to the high chemical reaction activity of metallic sodium, it easily reacts with organic electrolyte, forming an uneven and unstable solid electrolyte interface layer (SEI) on its surface, causing a series of problems It mainly includes: i) The volume of metallic sodium undergoes huge expansion changes during electrochemical cycling, and ii) dendrite growth due to uneven sodium deposition. The above problems can lead to rapid degradation of electrode performance and battery short-circuit problems, thus It severely hinders the wide application of sodium anode.
Recently, the research group of Professor Weiyang Li from Dartmouth College in the United States has grown 1D Na3Ti5O12 nanowires in-situ on the Ti3C2 MXene material layer and on the interface through simple CTAB intercalation and NaOH oxidation, and prepared 1D/2D Na3Ti5O12 /MXene hybrid nanomaterials. Due to its unique 1D/2D hybrid nanostructure, the Na3Ti5O12-MXene hybrid nanostructure shows unique advantages as a metal Na negative electrode substrate material: (1) Due to CTAB intercalation and interface stabilization, Na3Ti5O12 nanowires have uniform and long dimensions The structure exhibits excellent Na+ diffusion characteristics and Na+ reactivity, which helps promote the chemical interaction between deposited Na and the substrate material (Na3Ti5O12 + xNa = Na3+xTi5O12), providing abundant Na nucleation sites; (2 ) The interconnected nanochannels formed by the in-situ combination of 1D nanowires and 2D nanosheets can effectively control and limit the nucleation and growth of Na.
Use CTAB to intercalate Ti3C2 MXene, and then use NaOH oxidation treatment to prepare 1D/2D Na3Ti5O12/MXene nanocomposite. The research results show that after the CTAB intercalation treatment, the reactant NaOH solution is easily embedded between the Ti3C2 layers, which more effectively promotes the subsequent growth of Na3Ti5O12 nanowires. In the subsequent oxidation process, the NaOH solution embedded between the MXene layers gradually oxidized the Ti-C bond on the Ti3C2 MXene interface to form Na3Ti5O12 nanowires. (CTAB can stabilize the interface of the Na3Ti5O12 nanowires in this process, so that the nanowires The size is more uniform and longer), and finally a unique 1D/2D Na3Ti5O12/MXene hybrid structure nanomaterial is obtained. The microstructure analysis and electrochemical performance evaluation of the obtained materials confirmed that the 1D/2D Na3Ti5O12/MXene hybrid nanostructures obtained after CTAB pretreatment have more uniform and long Na3Ti5O12 than the hybrid nanostructures without CTAB treatment. Nanowires, when used as metal Na substrate materials, have richer Na nucleation sites, and more effectively control and limit Na nucleation and growth. At high current density (10 mA cm-2) and high capacity (20 mAh) cm-2) showed stable cycle performance under the test conditions. The article was published in Nano Letters, the top international journal in the nano field. The corresponding author of the paper is Professor Weiyang Li; the first author is postdoctoral fellow Luo Jianmin.


Figure 1. Schematic diagram of Na nucleation, deposition and circulation in Cu and 1D/2D Na3Ti5O12-MXene substrate materials
As shown in Figure 1b, using electrochemically active 1D/2D hybrid nanostructures as metal Na substrate materials, the “hot spots” that are conducive to dendritic growth generated during the nucleation and growth of metal Na can be effectively suppressed, so Na+ The flow distribution is more uniform and promotes the uniform deposition of metal Na. As for the Cu current collector (Figure 1a), the higher local current density easily leads to uneven nucleation and random deposition of metallic Na, which ultimately leads to the formation of "hot spots" in the protruding area, so Na dendrites grow.
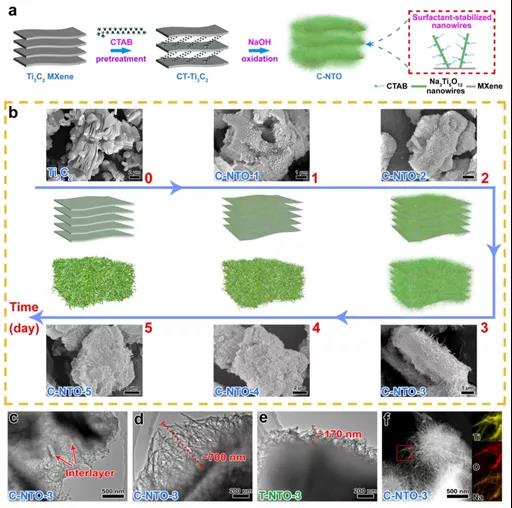
Figure 2. (a) Flow chart of preparation of 1D/2D Na3Ti5O12-MXene (C-NTO) derived from CT-Ti3C2. (b) Schematic diagram of morphology evolution of C-NTO with oxidation time. (c,d) CT -TEM image of C-NTO-3 derived from Ti3C2. (e) TEM image of T-NTO-3 derived from Ti3C2. (f) STEM image of C-NTO-3 and EDS mapping of Ti, O and Na
As shown in Figure 2a, the Ti3C2 MXene (CT-Ti3C2) pretreated by CTAB, in the subsequent NaOH oxidation process, because CTAB has a stabilizing effect on the interface of the nanowires, CT-Ti3C2 is more conducive to uniform growth The slender Na3Ti5O12 nanowires finally result in a unique 1D/2D Na3Ti5O12/MXene hybrid structure nanomaterial. The above conclusion is also confirmed by the corresponding TEM images (Figure 2c-d are TEM images of CT-Ti3C2-derived 1D/2D hybrid nanostructures; Figure 2e is the TEM image of Ti3C2-derived nanostructures). The 1D/2D Na3Ti5O12-MXene composite structure nanomaterial derived from CT-Ti3C2 has a layered structure of obvious combination of 1D and 2D, and the Na3Ti5O12 nanowires on the outer surface are also very uniform, and the length reaches 700 nm, which is longer than that derived from Ti3C2 The nanowires (170 nm) in the composite nanostructure are more uniform and longer.

Figure 3. (a) The corresponding Na deposition curve when C-NTO-3 is used as the substrate. (b) The corresponding schematic diagram and reaction equation of Na when the C-NTO-3 substrate is deposited. (c) Before and after the deposition of Na C-NTO- 3 The change of Ti valence state in the substrate. (dj) Na deposition diagram on C-NTO-3 substrate, and the corresponding ex-situ SEM image
As shown in Figure 3a-c, when C-NTO-3 is used as the substrate material, during the Na deposition process, XPS results confirm that Na will chemically interact with the substrate (Na3Ti5O12 + xNa = Na3+xTi5O12), which is Na nucleation Provides a wealth of sites. In addition, comparing the SEM images of C-NTO-3 before and after Na deposition, it is found that the interconnected nanochannels formed by the in-situ combination of 1D nanowires and 2D nanosheets can effectively control and limit the nucleation and growth of Na.
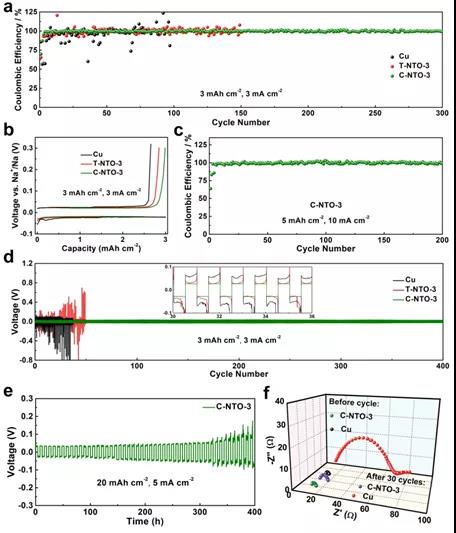
Figure 4. Corresponding electrochemical performance of 1D/2D Na3Ti5O12-MXene (C-NTO-3) derived from CT-Ti3C2 as metallic Na substrate
As shown in Figure 4, CT-Ti3C2-derived 1D/2D Na3Ti5O12/MXene (C-NTO-3) has more uniform and longer Na3Ti5O12 nanowires than Ti3C2-derived hybrid nanostructures (T-NTO-3). Therefore, when used as a metal Na substrate material, it has more abundant Na nucleation sites, and more effectively controls and restricts the nucleation and growth of Na. At high current density (10 mA cm-2) and high capacity (20 mAh cm-2) ) Shows stable cycle performance under the test conditions
 Figure 5. Full cell configuration and electrochemical performance of C/S//C-NTO-3/Na and NVP//C-NTO-3/Na
Figure 5. Full cell configuration and electrochemical performance of C/S//C-NTO-3/Na and NVP//C-NTO-3/Na
As shown in Figure 5, using C-NTO-3/Na as the negative electrode material, C/S positive electrode material and Na3V2(PO4)3 positive electrode material to assemble a full battery, it has more excellent performance than the full battery assembled with pure Na negative electrode material. The electrochemical performance also highlights the role of the 1D/2D Na3Ti5O12-MXene composite nanostructure as a metal Na substrate, which is conducive to the formation of stable SEI on the metal Na surface and inhibits the formation of Na dendrites and dead Na.
Literature link
Jianmin Luo, Xuan Lu, Edward Matios, Chuanlong Wang, Huan Wang, Yiwen Zhang, Xiaofei Hu, and Weiyang Li* Nano Lett. 2020, DOI: 10.1021/acs.nanolett.0c03215.
Source: MXene Frontier
This information comes from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately.











 Figure 5. Full cell configuration and electrochemical performance of C/S//C-NTO-3/Na and NVP//C-NTO-3/Na
Figure 5. Full cell configuration and electrochemical performance of C/S//C-NTO-3/Na and NVP//C-NTO-3/Na