


Over the past few years, research into next-generation battery technologies (beyond lithium-ion batteries) has advanced rapidly. A key challenge for these emerging batteries is the lack of suitable electrode materials, which severely limits their further development. MXenes, as a new class of 2D transition metal carbide/nitride/carbides, is considered as a potential electrode material for these emerging batteries due to some of its desirable properties. Large and controlled layer spacing, excellent hydrophilicity, ultra-high electrical conductivity, synthetic diversity, and rich surface chemistry make MXenes a promising electrode material, as well as other parts of emerging batteries. Recently, Professor Husam N. Alshareef of King Abdullah University of Science and Technology in Saudi Arabia published an overview paper titled MXenes for Recommissioning Beyond the Lithium-ion in the international top Materials journal Advanced Materials. It summarizes the research progress in the commissioning of MXenes in various Rechargeable Batteries, including alkaline metal Ion Batteries (Na+, K+) and multivalence Ion Batteries (Mg2+, Zn2+ and Al3+). In particular, the synthesis strategy and MXenes play different roles in the battery, such as electrode, metal negative protective layer, sulfur host, diaphragm improvement and conductive additive, etc., and the prospect of future development is also presented.


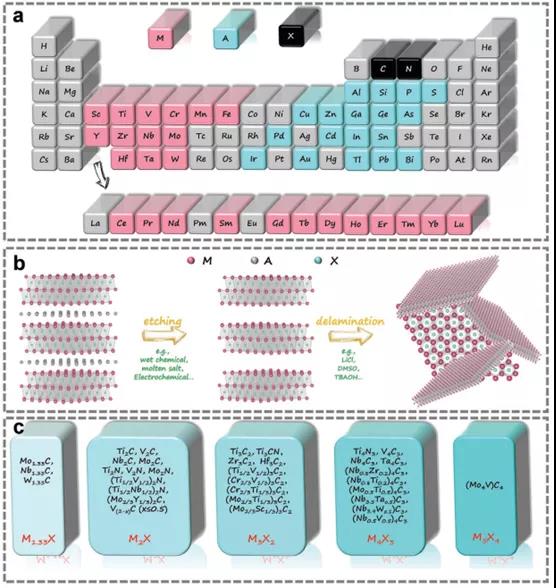
Figure 1. Composition of MAX and MXene.
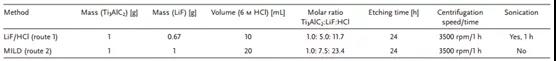
Table 1. Two MXenes synthesis methods and experimental parameters.

Figure 2. Different etching methods.
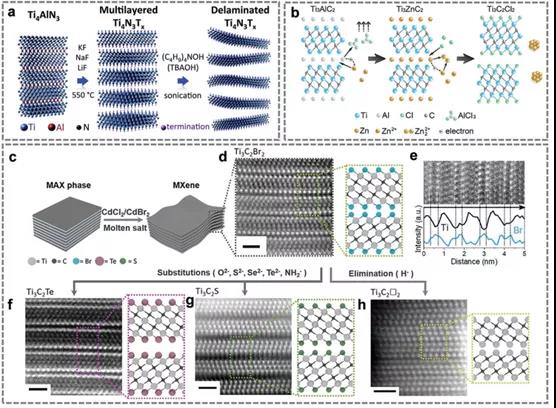
FIG. 3. Molten salt etching of Ti4N3Tx; ZnCl2 molten salt etching Ti2C2Cl2; The MAX phase is etched by molten Salts of Lewis acid.
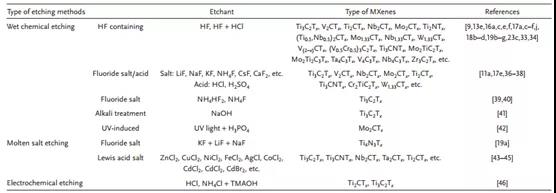
Table 2. Summary of different etching methods.
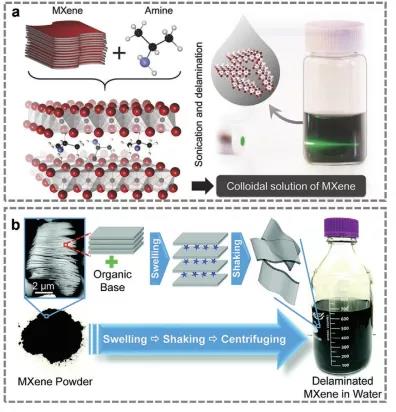
Figure 4. Synthesis of Nb2CTx.
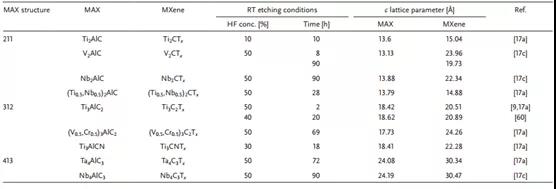
Table 3. Summary and comparison of lattice parameters of different MAX phases and MXenes.

Figure 5. Theoretical calculation of MXenes in the application of lithium sulfur battery.
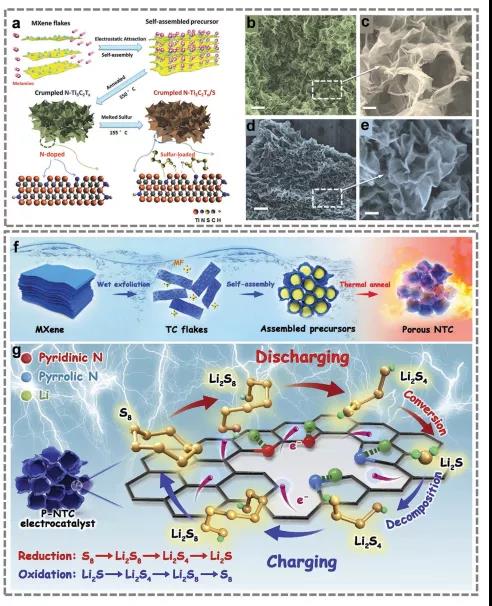
FIG. 6. Preparation process of N-TI3C2Tx@S electrode.
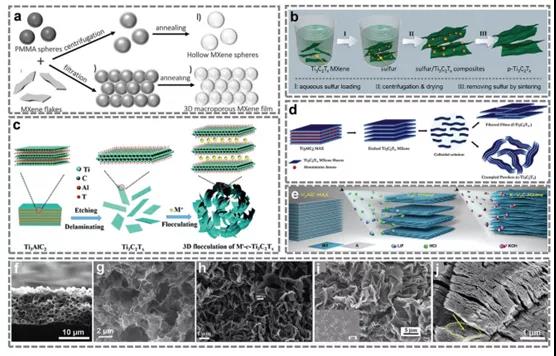
Fig.7 preparation process of porous and folded MXene on 3D.
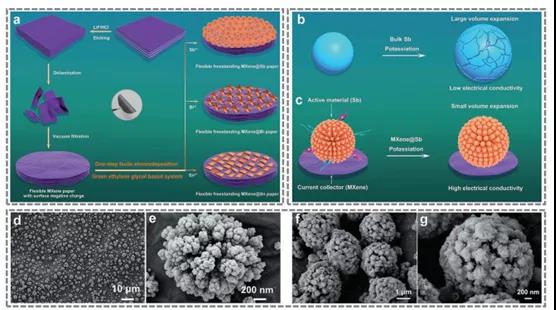
Figure 8. MXene@ metal (Sb, Bi and Sn) flexible self-supporting electrode.
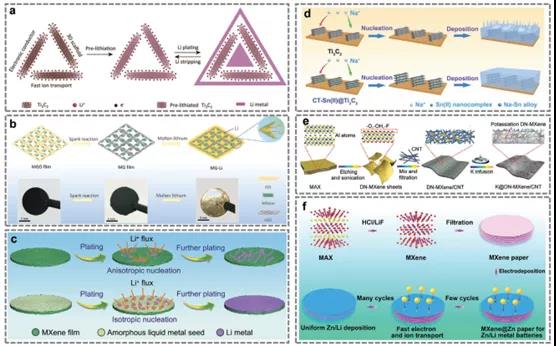
FIG. 9. Preparation of some typical MXene based electrodes.

The high electrical conductivity and rich surface chemistry of MXenes materials have great potential to play different roles in batteries. Although great progress has been made in MXenes research in recent years, some challenges remain that need to be addressed by researchers.
1) Currently, HF is used in the main synthesis methods of MXene, which is harmful and toxic to experimenters. The exploration of low-cost, efficient and environmentally friendly etching methods and the use of other etching agents that are safer than HF are particularly important for the preparation of large-scale high-quality MXene. Among them, Lewis acid melting salt method and electrochemical method are very promising. In addition, the synthesis method will also affect the electrical conductivity of MXene materials, and the exploration of new etchants that have a small impact on the environment can further improve the electrical conductivity of MXene.
On the other hand, the bottom-up method (such as CVD) can effectively directly synthesize metal carbide, so it may also be a promising new method for the synthesis of high-quality MXene.
2) The rich surface chemistry of MXenes has an important impact on electrochemical properties. These functional groups can selectively react with specific compounds, thus affecting energy storage performance. The control of surface functional groups remains a challenge. Careful choice of etchant and etching method can help.
3) MXenes usually suffers from rapid oxidation in air and water. On the one hand, surface oxides increase electrochemical activity, while on the other hand, their electrical conductivity decreases, and the study of the intrinsic properties of MXene brings complexity.
4) MXene based electrodes usually exhibit pseudocapacitance characteristics. Because of their high electrical conductivity, MXene electrodes usually exhibit excellent multiplier performance and are ideal for hybrid ion capacitors.
5) Although MXenes has been deeply studied in the field of energy storage, the ion dynamics and charge storage behaviors of different energy storage devices (especially K+/Zn2+/Al3+ batteries, etc.) are not comprehensive enough. This is helpful to the design and optimization of MXene based electrode.
6) Although more than 30 MXenes have been synthesized in the laboratory, the most intensively researched MXene, Ti3C2Tx, is still the first MXene, although it shows unique and potential characteristics, it does not mean that it is the best choice for all applications.
Literature link:
DOI: 10.1002 / adma. 202004039
Source: MXene Frontie
This information is from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately





















