
The use of sustainable, green chemical principles to produce multifunctional biological materials is of great benefit to the development of the environment. In the design of biological materials, the use of catalytic reactions combined with raw materials from biomass can promote the development of environmentally friendly technologies and sustainable materials. The combination of two catalytic cycles (combined catalysis) was revealed, including oxidative decarboxylation and quinone-catechol redox catalysis, a multifunctional antibacterial hydrogel based on engineered lignin. This biologically inspired design mimics the catechol chemistry used by marine mussels in nature. In this direction, the team of Professor Samson Afewerki from Uppsala University in Sweden published a study titled "Combined Catalysis for Engineering Bioinspired, Lignin-Based, Long-Lasting, Adhesive, Self-Mending, Antimicrobial Hydrogels" on ACS Nano. Sex thesis. The author studied two catalytic cycle combinations (combined catalysis) of lignin-based multifunctional antibacterial hydrogels, including oxidative decarboxylation and quinone-catechol redox catalysis (Figure 1).
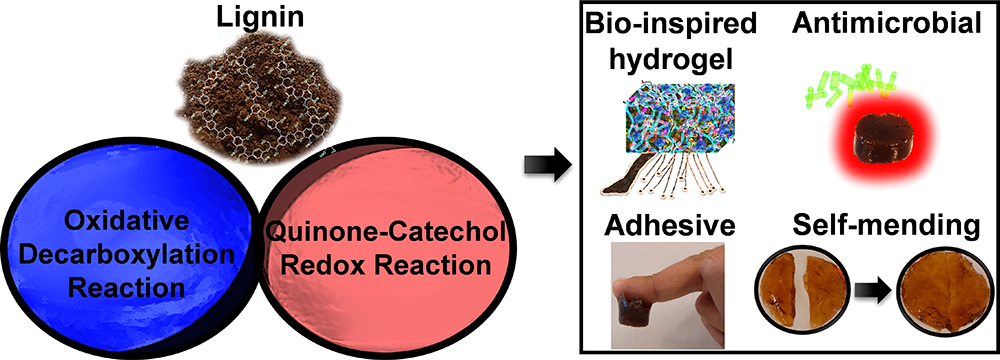
Figure 1 Design ideas
First, the author combines two different catalytic reaction cycles (combined catalysis) to design a lignin-based, tough, adhesive, self-healing, antimicrobial hydrogel. At the same time, lignin can be used as a sustainable material to prepare various metal-based nanoparticles (NPs) by acting as a reducing agent and stabilizer to produce NPs and activate the catechol function of lignin (Figure 2).

Figure 2 Overview of catalytic chemistry strategies
However, there is a limitation that lignin and corresponding quinones have the ability to scavenge free radicals. When this process occurs in a sufficient amount, the intermolecular cross-linking is likely to be inhibited, resulting in the inability to form a free-standing hydrogel. After that, the author conducted a thorough verification and screening of this idea to design a cross-linkable hydrogel with tissue adhesive properties. The initial cross-linking attempts used PAA and free radical initiator APS or catalyst silver nitrate (AgNO3). None of these provided any successful cross-linking self-fixing hydrogels. Although APS generates free radicals, it does not have a regeneration environment or catalyst. Under the circumstances, their life span is usually very short, causing the persulfate free radicals to react with water to decompose. When studying other compounds containing polymer carboxyl groups, such as alginate and carboxymethyl cellulose (CMC), cross-linked hydrogels were not successfully prepared, which may be the result of fewer carboxyl groups in their structure (Figure 3 ).

Figure 3 Optimization study of engineering multifunctional hydrogel
Next, the author conducted a thorough characterization of the engineering materials. Fourier transform infrared spectroscopy (FTIR) analysis was performed on samples of lignin 5AgNPs and lignin 1.25AgNPs-PAA. UV-Vis spectroscopy analysis of the generated lignin-based AgNPs confirmed the formation of quinones and the formation of AgNPs, and with the increase of the silver addition in the reaction mixture, the generation of AgNPs increased, indicating that the higher silver addition promotes the lignin The catechol on the element reacts with methoxy oxygen to form a quinone group. Scanning transmission electron microscopy (STEM) analysis of lignin 5AgNPs showed that AgNPs were uniformly distributed with a diameter of about 10 nm. AgNPs were also detected in the corresponding hydrogel (lignin 1.25AgNPs-PAA), without changing its shape or The size indicates that the light oxidative decarboxylation crosslinking step has no negative effect on the morphology of AgNPs (Figure 4).

Figure 4 Characterization of lignin AgNPs-based materials
After that, the author used oscillation scanning (oscillation stress is 0.1 ~ 1000 Pa, frequency is 1 Hz), angular frequency (ω) is 0.1 ~ 100 rad s−1 and 10 pa, recording storage (G) and loss modulus (G) , Further evaluated the viscoelastic properties of the crosslinked hydrogel. The G value of each sample has nothing to do with stress, but the lignin 1.25 AgNPs-PAA and lignin 2.5 AgNPs-PAA samples show frequency dependence. These phenomena can also be observed in the cyclic compression test. The lignin-based hydrogel still maintains its original structure after 6 consecutive cycles of compression. At the same time, the toughness and high resilience of the lignin-based hydrogel are consistent with the internal hydrogel. Interpenetrating networks that interact through various covalent and non-covalent bonds are related (Figure 5).
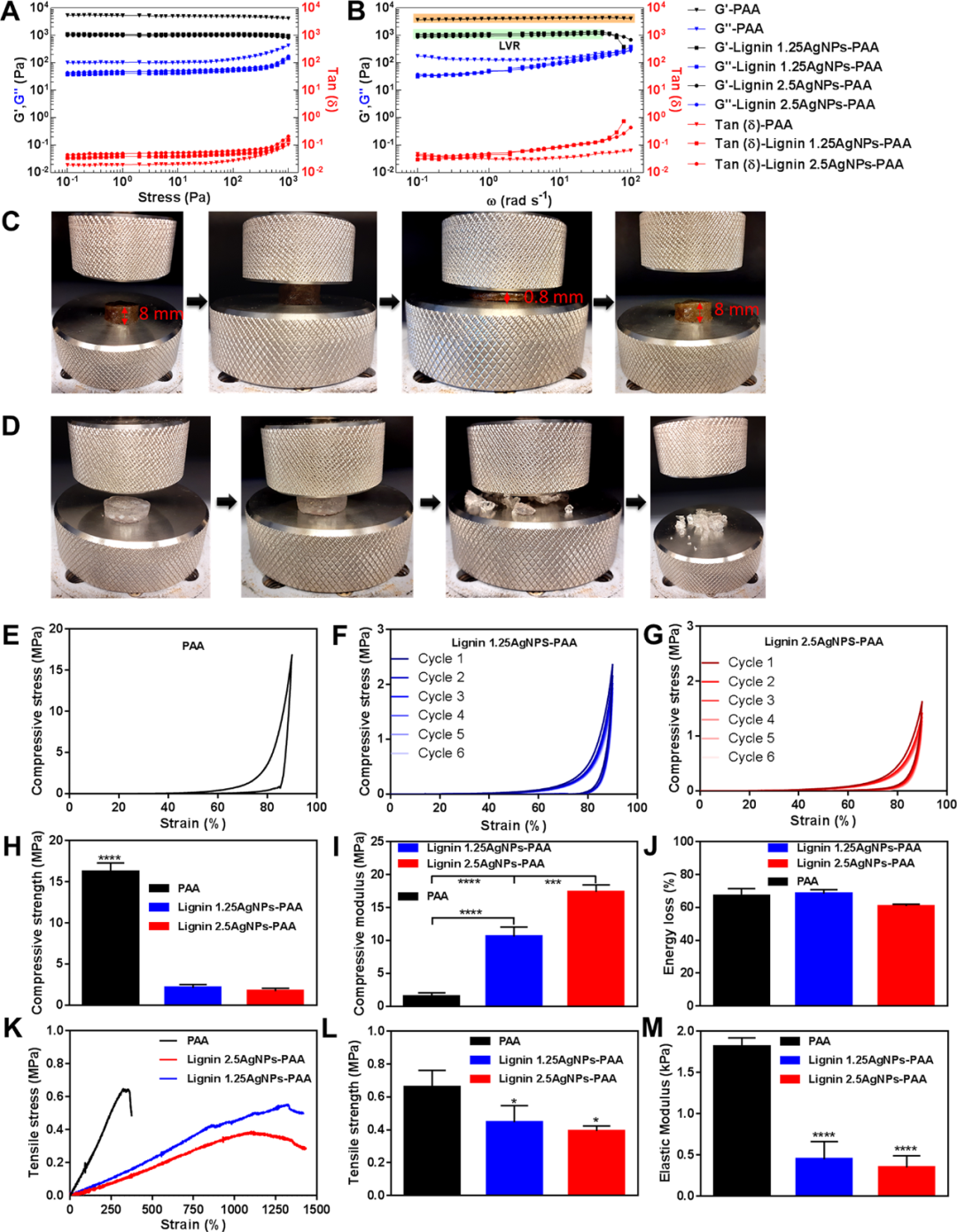
Figure 5 Rheology and mechanical properties of various engineering hydrogels designed
The incorporation of activated lignin enables the quinone-catechol redox reaction to promote physical interaction, thereby providing recoverability and also promoting self-repairing ability. After cutting the gel and bringing the pieces into contact with each other again, they immediately fuse back to their original structure. Preliminary screening results showed that the incorporation of activated lignin enhanced the tissue adhesion performance of the hydrogel formulation, and at the same time, the toughness and elasticity of the lignin-based hydrogel were verified by stretching the lignin-based hydrogel. The above test results illustrate some of the chemical interactions between the designed hydrogel and various surfaces, including the bonding of hydrogen, imines and amides to tissues, hydrophobic and π-π interactions, adsorption on metal surfaces, and metal The formation of complexes and electrostatic interactions that promote strong adhesion (Figure 6).
Figure 6 Self-repairing and bonding performance evaluation
Finally, the author used Gram-positive Staphylococcus aureus (S. aureus) and Gram-negative Escherichia coli (E. coli) to evaluate the antibacterial activity of the engineered hydrogel. Compared with the control group, all hydrogels showed antibacterial activity (the antibacterial activity of PAA hydrogels may be the result of the presence of Ag+ in the hydrogel). The results show that the engineering material has a significant inhibitory effect on both Gram-positive bacteria and Gram-negative bacteria. Using 3T3 fibroblasts as the experimental object, the hydrogels were treated with increasing concentrations (0.001-1000μg/mL) of PAA, lignin 5AgNPs, lignin 10AgNPs, lignin 1.25AgNPs-PAA, and lignin 2.5AgNPs-PAA to evaluate its Biocompatibility (Figure 7).

Figure 7 Antibacterial activity of hydrogel against Staphylococcus aureus and Escherichia coli
In this study, the author proposed a simple, co-catalyzed, environmentally friendly engineering multifunctional lignin-based antibacterial hydrogel. The basic principles of the design and the results of various experiments confirm that activating these two catalytic cycles can form a multifunctional hydrogel. Utilizing the incorporation of lignin and the oxidation-reduction reaction of quinone-catechol, the self-repairing properties of the hydrogel and the adhesion between different surfaces are produced. In particular, it is worth mentioning that the application of this technology has significant potential in the sustainable design of polymerization systems, which can be cross-linked to form hydrogels through simple and green methods. This method also provides a reference for the design of multifunctional hydrogels for other combined catalytic systems. These hydrogels may be potential candidates for various applications, including flexible electronic systems and dressings for infected wounds.
- Long press the QR code to read the original English text -
Paper link:
https://doi.org/10.1021/acsnano.0c06346
Information source: EngineeringForLife
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately











