MXene is a transition metal carbide or carbonitride with a two-dimensional structure. The first MXene was composed and reported in 2011 by Professor Yury Gogotsi of Drexel University in the United States. It is relatively young in todays very hot two-dimensional materials. Since 2011, the MXene family has been continuously expanded. At the same time, due to its layered structure, surface hydrophilicity and metal conductivity (> 6000 S cm-1), it has been used in the fields of supercapacitors, batteries, battery shielding, composite materials and catalysis All of the applications have achieved great success (click to read related). In recent years, some theoretical studies have also predicted some of the more interesting properties of MXene, such as the opening and closing of the band gap, room temperature electron mobility over 10000 cm2/V, adjustable work function, semi-metallicity, two-dimensional ferromagnetism, etc. However, the novel properties predicted by these theories require precise control of the surface chemistry of MXene, which cannot be achieved by the current mainstream MXene preparation methods.
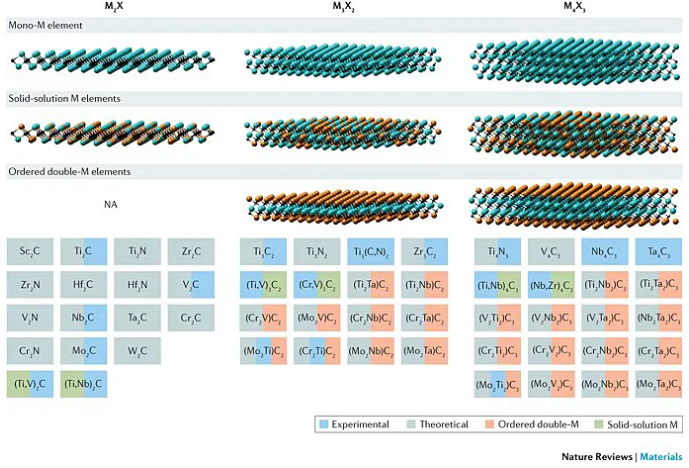
Figure 1. MXene reported in early 2017. Image source: Nat. Mater. [1]
The current mainstream preparation method of MXene is still the process of etching the bulk MAX phase with hydrofluoric acid solution explored by Professor Yury Gogotsi. The MAX phase is a type of layered ceramics with excellent ductility, where M represents a transition metal, A represents a 13th or 14th group element, and X represents carbon or nitrogen. Taking the earliest reported preparation of Ti3C2 (MXene) from Ti3SiC2 (MAX phase) as an example, the hydrofluoric acid solution selectively etches away each layer of silicon atoms, leaving Ti3C2 with a layered structure.
Although the etching process with hydrofluoric acid solution or other fluorine-containing solutions was successfully used in the later development of MXene and became the standard MXene preparation method, this method is not only toxic, harmful, and corrosive. In addition to the extremely strong performance, more importantly, the obtained MXene has always had the problem of uncontrollable surface chemistry. Because of this, the MXene prepared by this method is usually abbreviated as MXTx, where Tx stands for terminal groups such as F, O and OH with uncertain content; and this process is only suitable for MAX that can react with hydrogen fluoride, especially A It is the MAX phase of aluminum.
Exploring new MAX etching processes has also become an important direction of MXene research. At the beginning of 2019, Huang Qings team from the Advanced Energy Materials Engineering Laboratory of Ningbo Institute of Materials, Chinese Academy of Sciences reported a new MXene preparation process on JACS [2]. They noticed that Ti3AlC2 (MAX phase) reacts similarly to hydrofluoric acid in molten ZnCl2 (Lewis acid). After in-depth research, they obtained a series of Mn+1ZnXn phases through displacement reactions, and a new type of surface with Cl MXene (Mn+1XnCl2).
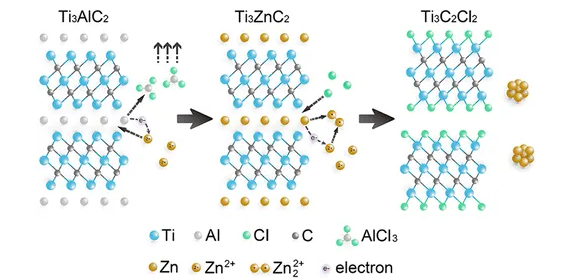
Figure 2. Molten zinc chloride is used for stripping to prepare MXene. Image source: JACS [2]
On this basis, the Huang Qing team carried out in-depth and systematic research. They proposed that the oxidation-reduction potential of the cation in the molten salt and the A element in the MAX phase can be used as a criterion for peeling. Taking Ti3SiC2, which was difficult to peel off from fluorine-containing solutions in the past, as an example, in the copper chloride molten salt at 700 ℃, the redox potential of Cu/Cu2+ is -0.43 eV, and the redox potential of Si/Si4+ is -1.38 eV. Therefore, the ionic Cu2+ in the molten salt can easily oxidize Si atoms to Si4+, Si4+ eventually forms SiCl4 gas with Cl- and escapes from the Ti3C2 sublayer, while Cu2+ is reduced to Cu element. The residual Cu in the product can be removed by ammonium persulfate solution and other methods, and finally Ti3C2Tx (Tx = Cl, O) MXene containing Cl and O mixed functional groups on the surface.
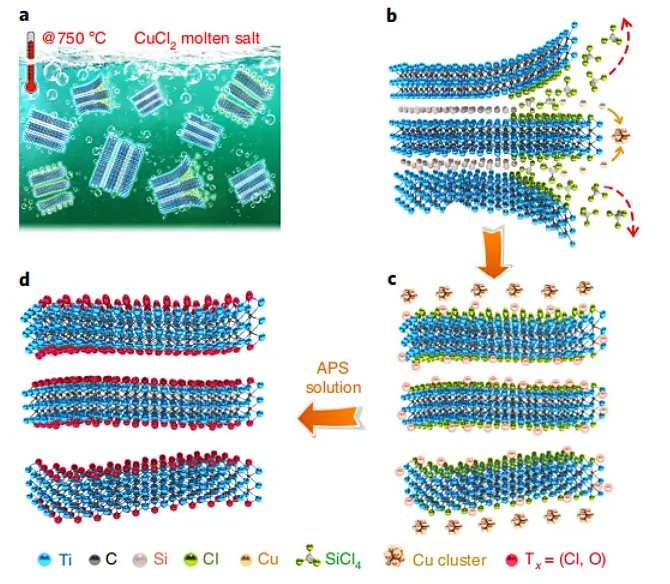
Figure 3. Preparation of MXene by stripping molten salt. Image source: Nat. Mater. [3]
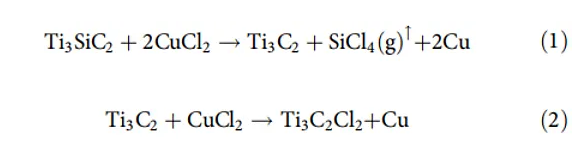
Figure 4. The chemical process of peeling molten salt to form MXene. Image source: Nat. Mater. [3]
Under the guidance of this redox potential criterion, Huang Qing’s team further used a variety of molten salts (CdCl2, FeCl2, CoCl2, CuCl2, AgCl, NiCl2) for Ti2AlC, Ti3AlC2, Ti3AlCN, Nb2AlC, Ta2AlC, Ti2ZnC, Ti3ZnC2, etc. MAX The phase is stripped to obtain the corresponding MXene.
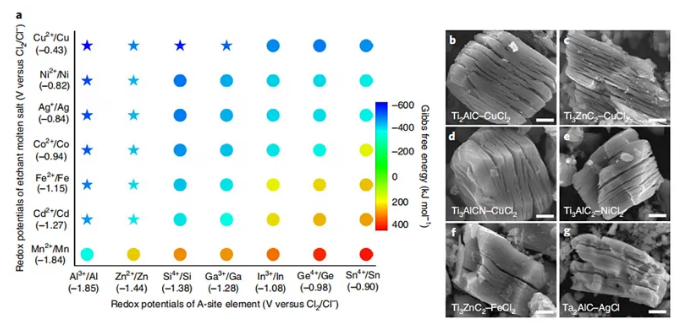
Figure 5. The versatility of molten salt stripping to prepare MXene. Image source: Nat. Mater. [3]

Furthermore, they cooperated with Professor Simon Patrice of the University of Toulouse in France and researcher Lin Zifeng of Sichuan University to use the Ti3C2 MXene obtained by stripping off Ti3SiC2 from CuCl2 molten salt as the negative electrode material, and achieve up to 738 C g-1 (205 mAh) in 1 M LiPF6 electrolyte. g-1) lithium storage capacity, and has a large voltage window of 2.8 V (0.2 to 3 V vs. Li+/Li), which is very promising in electrochemical energy storage devices.

The above results developed by Huang Qings team were published online on Nature Materials on April 13 this year [3]. A few months later, Science published another article from the University of Chicagos Dmitri V. Talapin team using molten salt to prepare a novel MXene research [4].
The Dmitri V. Talapin teams approach to MAX phase precursors is based on the research of Huang Qings team. In addition to treating the MAX phase with molten CdCl2 to obtain Ti3C2Cl2, Ti2CCl2 and Nb2CCl2 whose end groups are chlorine, they also used CdBr2 molten salt to prepare MXene (Ti3C2Br2 and Ti2CBr2) with bromine end groups that have never been reported. For these new MXenes, Dmitri V. Talapins team used high-resolution scanning transmission electron microscopy, Raman spectroscopy, and a series of X-ray methods to perform detailed structural characterization of the morphology, structure and composition of the resulting products.

Figure 6. Dmitri V. Talapin s team expanded the types of molten salts and carried out more extensive end-group transformation studies. Image source: Science [4]
In terms of end groups, the bond energy of the Ti-Cl bond (-804 kJ/mol) or Ti-Br bond (-617 kJ/mol) in the new MXene is compared to the Ti-F (-1649 kJ) in the classic MXene. /mol) or the Ti-O bond is much weaker. Dmitri V. Talapins team explored the end group conversion chemistry. For example, they disperse Ti3C2Br2 in molten CsBr/KBr/LiBr and add Li2Te or Li2S to obtain Ti3C2Te and Ti3C2S, respectively. Similarly, they also converted Ti3C2Cl2 into Ti3C2Te, Ti3C2S and Ti3C2(NH). Interestingly, at 300 ℃, LiH can reduce Ti3C2Br2 and Ti2CBr2 to Ti3C2□2 and Ti2C□2 (where □ stands for defects). Further treating the above-mentioned sample with n-butyl lithium can obtain a lithium intercalation product, which can be further dispersed in a polar organic solvent to form a monolayer product.
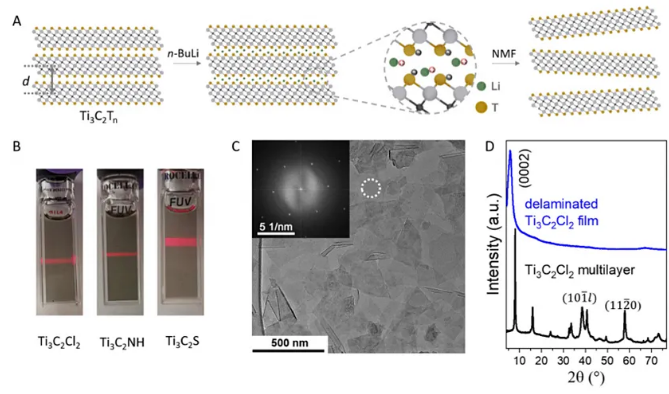
Figure 7. Single-layer dispersion of MXene achieved by n-butyllithium treatment. Image source: Science [4]
Through XRD monitoring, they found that the end group conversion process was accompanied by an increase in the layer spacing. And further modeling analysis showed that during the above process, the lattice constants such as the Ti-Ti bond length in MXene changed. Combined with high-resolution STEM, they confirmed that the process of Br/Cl atom exchange to form Te/S is accompanied by in-plane stretching (up to 18%) and compression in the c-axis direction.

Figure 8. The effect of end group modification on the lattice. Image source: Science [4]
The huge change in the lattice structure means that the surface modification will have a huge impact on all aspects of the performance of MXene. The Dmitri V. Talapin team investigated the electron transport behavior of the Nb2C series MXene and found that they exhibit superconducting properties affected by the end groups. For example, the superconducting transition temperature of Nb2CCl2 is about 6 K, while bulk Nb2AlC does not possess superconducting properties.
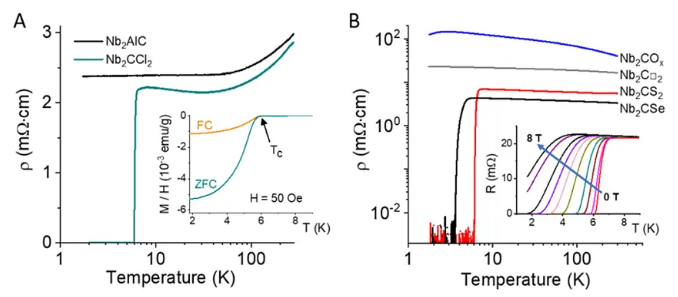
Figure 9. Superconducting properties significantly affected by end groups. Image source: Science [4]
In summary, Huang Qings team pioneered and developed a strategy to prepare MXene by stripping the MAX phase from molten salt, avoiding the use of harmful hydrofluoric acid and enriching the types of MXene. They studied the mechanism of this process in depth, and at the same time used the new MXene obtained in high-performance electrochemical energy storage. The Dmitri V. Talapin team focused on studying the end-group chemistry of MXene obtained by this strategy, and on this basis, studying the electronic transport properties of the material.
Reference materials: 1. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater., 2017, 2, 16098. https://www.nature.com/articles/natrevmats2016982. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc., 2019, 141, 4730-4737 https://pubs.acs.org/doi/10.1021/jacs.9b005743. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater., 2020, 19, 894–899 https://www.nature.com/articles/s41563-020-0657-04. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science, 2020, DOI: 10.1126/science.aba8311https://science.sciencemag.org/content/early/2020/07/01/science.aba8311
Information source: X-MOL information
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately