 MXene is a new type of two-dimensional layered carbon/nitride discovered in recent years. Its unique two-dimensional layered structure, tunable surface chemistry and conductivity make it useful in energy storage, catalysis, electromagnetic absorption/shielding, Composite materials and sensors have shown good application prospects. MXenes are usually made by selectively etching the A atomic layer of the Mn+1AXn phase precursor, and its general chemical formula is Mn+1XnTx. Numerous theoretical studies have shown that the type and position distribution of Tx groups on the surface of MXene have important effects on its energy band structure, electrical conductivity, magnetic properties, and thermal stability. However, most of the early MXene materials were obtained by etching the MAX phase with a fluorine-containing etchant, so the group types are limited to the three types -OH, -O, and -F. Although researchers have made many explorations, the development of new surface groups and their composition regulation is still an experimental problem.
MXene is a new type of two-dimensional layered carbon/nitride discovered in recent years. Its unique two-dimensional layered structure, tunable surface chemistry and conductivity make it useful in energy storage, catalysis, electromagnetic absorption/shielding, Composite materials and sensors have shown good application prospects. MXenes are usually made by selectively etching the A atomic layer of the Mn+1AXn phase precursor, and its general chemical formula is Mn+1XnTx. Numerous theoretical studies have shown that the type and position distribution of Tx groups on the surface of MXene have important effects on its energy band structure, electrical conductivity, magnetic properties, and thermal stability. However, most of the early MXene materials were obtained by etching the MAX phase with a fluorine-containing etchant, so the group types are limited to the three types -OH, -O, and -F. Although researchers have made many explorations, the development of new surface groups and their composition regulation is still an experimental problem.
Researchers from the Advanced Energy Materials Engineering Laboratory of Ningbo Institute of Materials found that the MAX phases such as Ti3ZnC2 and Ti2ZnC have structural transformations in the ZnCl2 molten salt: that is, the Zn atoms located in the MAX phase A layer are attacked by the Zn2+ in the molten salt, from the A layer Pull away. The Cl- in the molten salt further enters the A layer and combines with the Mn+1Xn sublayer to form Mn+1XnCl2 (Ti3C2Cl2 and Ti2CCl2) structural units and dissociate along the interlayers to obtain a new MXene material with all Cl groups (Journal of the American Chemical Society, 2019, 141(11):4730-4737). The research team further established a Gibbs free energy mapping map for the oxidation-reduction potential/displacement reaction of cations and A elements in a high-temperature molten salt environment, and proposed a general strategy for synthesizing MXene using Lewis acid molten salt etching MAX phase (Nature Materials, 2020 , 19(8): 894). The team cooperated with the team of Professor Patrice Simon of the Third University of Toulouse, France, and the research showed that Ti3C2Tx MXene synthesized by molten salt etching showed excellent pseudocapacitor lithium storage performance and excellent rate performance in organic electrolyte. The Lewis acid molten salt stripping method to synthesize MXene material is different from the mainstream solution stripping (such as fluorine-containing solution etching), which greatly improves the chemical safety of the experimental process and reduces the difficulty and cost of waste liquid disposal. It is expected to further promote the use of MXene materials. Research progress in the fields of energy storage, catalysis and chemical engineering, and biological diagnosis has therefore received extensive attention. Recently, the team of Professor Dmitri V. Talapin of the University of Chicago in the United States further carried out the anion exchange of end groups on the basis of Lewis acid etching, and synthesized surface groups with oxygen, imide, sulfur, chlorine, selenium, bromine, tellurium, etc. The first Nb2CSe MXene with superconducting properties was obtained (Science, 2020, 369: 979–983).
The above research shows that the Lewis acid etching method has great advantages and space in the chemical control of MXene surface, and it is expected to further expand the application research of MXene physics and chemistry. Researchers from the Advanced Energy Materials Engineering Laboratory of Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences used this method to synthesize a variety of MXene materials with halogen groups, and realized the combination of different types of groups by adjusting the molten salt composition. As shown in Figure 1, the research team synthesized MXene with mono-halogen groups such as Br and I, as well as MXene materials with binary and ternary mixed groups such as ClBr and ClBrI. The team further cooperated with the team of Professor Chunyi Zhi from City University of Hong Kong to study the influence of surface groups on the electrochemical performance of MXene (Figure 2). The cooperative team assembled these MXene materials into the positive electrode of water-based zinc-ion batteries and found that the type of surface groups has a significant impact on the electrochemical performance of MXene. The Ti3C2(OF) obtained by the traditional HF acid etching process and the Ti3C2Cl2 discovered by the team in the early stage show typical pseudocapacitance behavior. No redox peak is observed in the CV curve. The flat and wide CV curve mainly comes from the charge and discharge process. The rapid intercalation/deintercalation of ions, and MXenes own components do not participate in the redox reaction required for energy conversion in the battery system. In contrast, obvious and sharp redox peaks were observed in the CV curves of Ti3C2Br2 and Ti3C2I2, corresponding to the reversible conversion of Br-1/Br0 and I-1/I0, respectively. MXene Ti3C2(BrI) and Ti3C2(ClBrI) with mixed groups have two pairs of redox peaks. The energy conversion reaction of the halogen group makes Ti3C2Br2 and Ti3C2I2 show a significant discharge platform. When the current density is 0.5A·g-1, their capacities are 97.6mAh·g-1 and 135mAh·g-1, respectively, while Ti3C2( OF) and Ti3C2Cl2 do not have a discharge platform, and their discharge capacities are only 46.5mAh·g-1 and 51.7mAh·g-1, respectively. In addition, the capacity and energy density of Ti3C2 MXene containing -Br or -I groups are close to twice that of Ti3C2(OF) and Ti3C2Cl2, and the additional contribution mainly comes from the discharge platform caused by -Br and -I groups. In addition, Ti3C2Br2 and Ti3C2I2 also show excellent rate performance and cycle stability. The research team further revealed the energy conversion mechanism of Ti3C2Br2 and Ti3C2I2 electrodes through Raman spectroscopy and X-ray photoelectron spectroscopy. Analysis shows that during the charging process, the Br-/I- ion in the negative valence state loses electrons and is oxidized to a zero valence state. In the discharge process, the above reactions proceed in the opposite direction. In the above process, the oxidation-reduction reaction of Br and I elements is restricted between the Ti3C2 layers, which effectively avoids the loss of halogen ions and ensures cycle stability. For Ti3C2Cl2 and Ti3C2(OF), the above reversible conversion reaction cannot be realized. It is precisely because of the state changes of different groups during charge and discharge that MXene exhibits completely different electrochemical behaviors. The work was recently published in the internationally renowned journal ACS Nano (DOI: 10.1021/acsnano.0c07972). The cooperative team further conducted in-depth research on the electrochemical properties of the iodine group MXene Ti3C2I2, and jointly reported a high-performance zinc-iodine battery based on Ti3C2I2 cathode, and achieved a series of iodine in water-based zinc batteries through the strategy of electrolyte regulation. The multivalent state transition of the positive electrode (Figure 3). Studies have shown that F- and Cl- ions in the electrolyte can activate and stabilize the transition of iodine to a normal valence state, and effectively promote the I0/I+ redox reaction. There are two stable discharge platforms in the corresponding battery, located at 1.7V and 1.45V, which greatly improves the high potential output capability of the zinc-iodine battery. Compared with the zinc sulfate system reference under the same conditions, the discharge capacity of the zinc-iodine battery of the new system is increased by 205%, and the energy density is increased by 330%. It is worth noting that the extra part is completely in the high voltage range, which greatly optimizes the effective output of the battery and broadens its application scenarios. In addition, because it is confined between the MXene layers, the loss of active materials is effectively suppressed, so the battery system exhibits good cycle stability, with a cycle life of more than 2,800 times. The cooperative team further used in-situ Raman characterization technology to reveal that the stabilizing effect of the free highly negative Cl- ions in the electrolyte on the positive iodide ions is the decisive factor to ensure that the reaction can proceed reversibly. This article was recently published in the internationally renowned journal Energy & Environmental Science (DOI: 10.1039/D0EE03086D). The above work was supported by the National Natural Science Foundation of China (51902320, 21805295), the Chinese Academy of Sciences International Cooperation and Exchange Project (174433KYSB20190019), Zhejiang Leading Innovation and Entrepreneurship Team (2019R01003), Ningbo Top Talent Program and other projects.
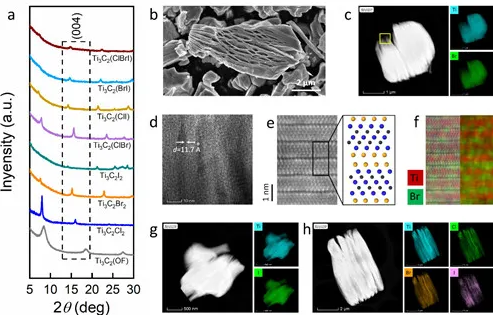
Picture 1 (a) XRD patterns of different surface groups Ti3C2 MXene; (b) SEM picture of Ti3C2Br2; (c) TEM picture and energy spectrum analysis showing the element distribution of Ti3C2Br2; (d) Ti3C2Br2 high-resolution transmission electron microscope Photo; (ef) High-resolution transmission electron micrograph and its energy spectrum analysis show the atomic occupancy of Ti3C2Br2; (gh) Transmission electron micrograph and energy spectrum analysis show that the element distribution of Ti3C2I2 and Ti3C2 (ClBrI) is different.
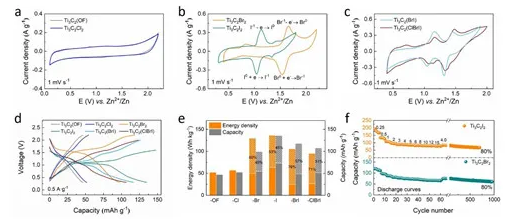
Figure 2 (ac) is different Voltammetric cycle curve of surface group Ti3C2 MXene; (d) constant current charge-discharge curve of Ti3C2 MXene with different surface groups; (e) comparison of capacity and energy density of Ti3C2 MXene with different surface groups; (f) Ti3C2Br2 and Picture of the cycle and rate performance of Ti3C2I2 electrode
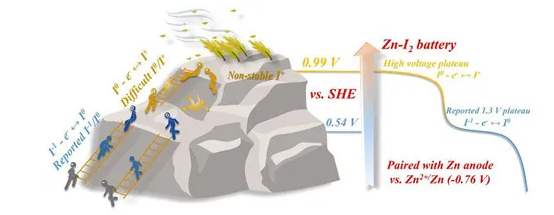
Figure 3 Schematic diagram of energy conversion mechanism of zinc-iodine battery based on Ti3C2I2 electrode
Information source: Ningbo Institute of Materials
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately







