 High-energy density intercalated lithium-ion battery cathode materials occupy a large share of the portable electronic equipment and electric vehicle market. The search for a new generation of environmentally friendly and abundant materials for economical and safe rechargeable lithium battery technology has attracted much attention.
High-energy density intercalated lithium-ion battery cathode materials occupy a large share of the portable electronic equipment and electric vehicle market. The search for a new generation of environmentally friendly and abundant materials for economical and safe rechargeable lithium battery technology has attracted much attention.
Introduction
Recently, Xiaoqing Yang, Enyuan Hu and Zulipiya Shadike (co-corresponding authors) of Brookhaven National Laboratory published a review entitled "Review on Organosulfur Materials for Rechargeable Lithium Batteries" in Materials Horizons. This article summarizes three organosulfur electrode materials recently reported: organic disulfide, polyorganosulfide and sulfide; synthesis, structural characterization and composition optimization of sulfurized polyacrylonitrile (SPAN) electrode; optimization of organic sulfur electrode electrochemistry Performance strategies; the application of liquid organic sulfur compounds in lithium-sulfur batteries and lithium metal batteries and their performance as SEI/CEI film-forming additives; finally, the development prospects of organic sulfur electrode materials and lithium-sulfur batteries are prospected.
Graphic guide
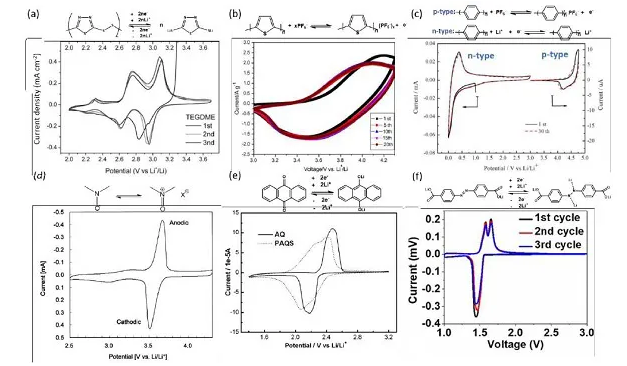
Figure 1. Cyclic voltammetry curve (a) PDMcT/PEDOT composite electrode, (b) Li/PTH battery, (c) PPP polymer electrode, (d) PTMA composite electrode, (e) AQ and PAQS electrode, (f) ADALS electrode
Organic electrode materials can be roughly classified into the following categories: organic sulfides, conductive polymers, organic radicals, carbonyl compounds, and azo compounds. Generally, the redox voltage of p-type compounds is higher than that of n-type compounds. For example, the redox potentials of conductive polymers, organic radicals and thioethers are 3.5 V, while the redox potentials of organic sulfides, azo compounds and carbonyl compounds are lower than 3.0. V. Voltage is an important factor affecting energy density. The theoretical capacity of organic sulfides is higher, and the capacity of azo compounds and carbonyl compounds is also higher than that of conductive polymers and organic free radicals. Electrical conductivity and kinetics are important factors that affect rate performance and electrochemical polarization. Compared with other organic compounds (such as conjugated carbonyl compounds and imide/azo compounds), the research on organic sulfide electrodes is still in its infancy.
1. Overview of organic sulfide electrode materials
Visco was equal to the first use of organic sulfide electrodes as energy storage materials in 1988, and systematically studied the charge and discharge behavior and ionic conductivity of different organic sulfide electrodes in sodium batteries. Although the organic sulfur electrode has poor cycle stability and large polarization, its advantages of low cost, low toxicity, low corrosion and low working temperature make it have a good application prospect in the field of energy storage.
1.1 Organic sulfide cathode material
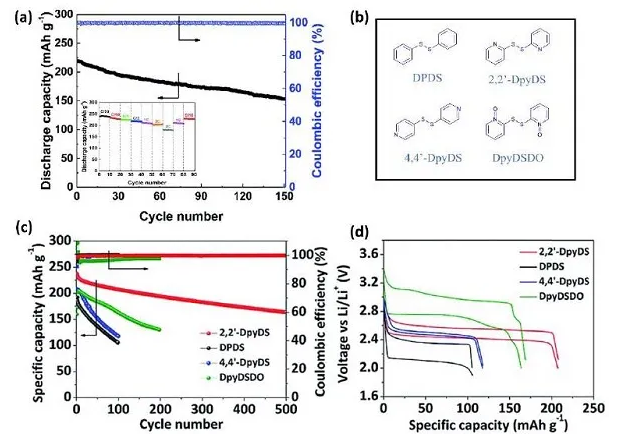
Figure 2. (a) Cyclic voltammetry and rate performance of DPDS@CNT positive electrode, (b) molecular structure, (c) cycle stability and (d) after 100 cycles of diphenyl disulfide and dipyridine disulfide Charge and discharge curve
The disulfide bond of the organic disulfide electrode can undergo a reversible redox reaction to provide charge and discharge capacity. In order to improve the kinetics and controllability of the DPDS electrode, Bhargav and others combined DPDS and CNT to prepare a DPDS@CNT self-supporting electrode, which exhibits excellent rate performance. In order to improve charge transfer, increase the utilization rate of active materials, and organic The overall performance of the material cathode provides a reference. Recently, Fu et al. further modified the molecular structure of DPDS, studied a series of bipyridine disulfides with similar capacities, and studied the relationship between their molecular structure and electrochemical performance. Although the molecular structures of these disulfides are similar, the charge-discharge behavior and cycle performance are quite different. The results show that when the phenyl group is substituted by a pyridine ring, the working voltage, energy efficiency and cycle stability of the organic disulfide are improved.
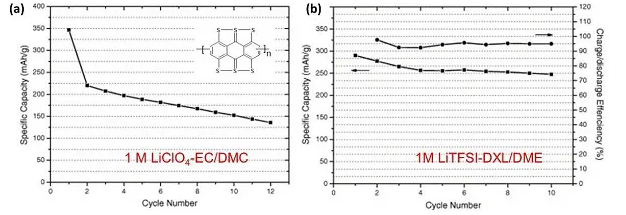
Figure 3. Cycle stability of PABTH electrode in (a) LiClO4-EC/DMC and (b) LiTFSI-DXL/DME electrolyte
The conjugated molecular structure promotes the electron transfer process in electrochemical reactions and improves the kinetics of organic sulfides. Therefore, organic molecules with both disulfide bonds and conjugated main chains have become an emerging research direction. A key problem of organic disulfide electrodes: the dissolution of monomer and/or discharge products in the electrolyte is the main cause of capacity degradation. The oxidative coupling reaction is used to synthesize ABTH homopolymer to improve the charge-discharge stability and electrical conductivity of ABTH. PABTH electrode in LiTFSI-DXL/DME electrolyte has higher reversible capacity and better cycle stability than LiClO4-EC/DMC, indicating that the electrochemical performance of organic disulfide is closely related to electrolyte, and ether electrolyte It inhibits the dissolution of organic disulfides.
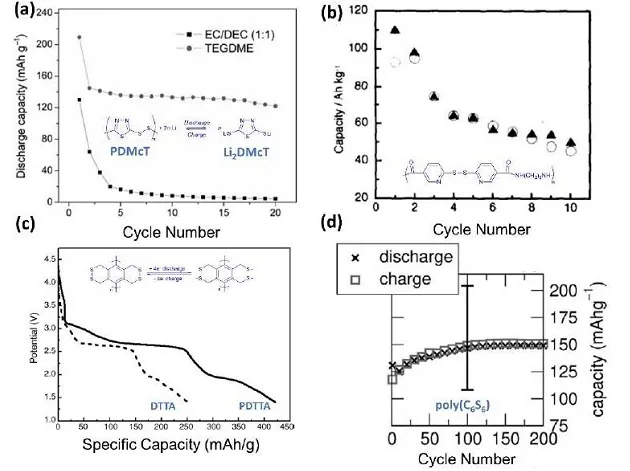
Figure 4. (a) The cycle stability of PDMcT/PEDOT electrodes in carbonate and ether electrolytes, (b) the changes in the capacity of 6,6-dithiadinicotinic acid and polyamide electrodes obtained from the CV peak area with the number of cycles , (C) The second circle discharge curve of DTTA and PDTTA, (d) The cycle curve of poly C6S6
Many studies have shown that the polymerization of organic disulfides can effectively inhibit sulfur loss during the cycle. Compared with the main chain type and side chain type organic disulfide polymer, the former is reduced to sulfide ions during the discharge process, and the discharge product is soluble in most organic electrolytes. Although organic disulfide compounds have a higher theoretical capacity, the actual capacity is still low. Organic sulfur compounds based on multiple S-S bonds were first proposed by Naoi et al. to increase the energy density of batteries.
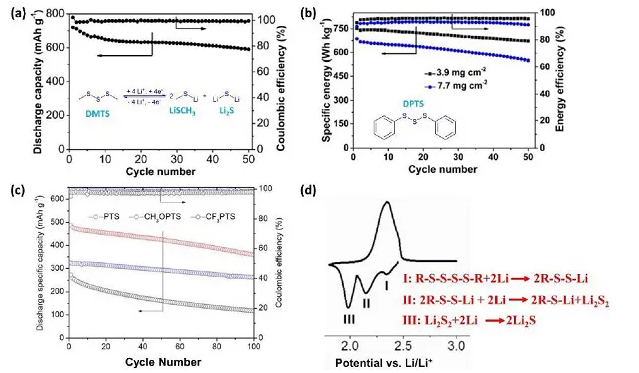
Figure 5. (a) Cyclic stability of DMTS, (b) Molecular structure of DPTS and its cyclic performance at different loading levels, (c) Cycle curves of PTS, CH3OPTS and CF3PTS, (d) CV diagram of PTS
In recent years, Fu et al. designed and synthesized a variety of organic trisulfide and tetrasulfide compounds, and successfully used them as cathode materials for lithium batteries. Through different characterization methods to study the capacity decay mechanism, the author found that the surface of lithium metal is passivated by sulfur-containing materials, indicating that the DMTS positive electrode has a polysulfide shuttle effect. In order to further improve the energy density, Guo et al. studied a series of PTS lithium battery positive electrodes and found that it is a 6-electron redox reaction. It shows that the physical and electrochemical properties of PTS electrode can be affected by adjusting functional groups, such as electron-donating group OCH3 and electron-withdrawing group CF3.
1.2 Vulcanized polyacrylonitrile electrode
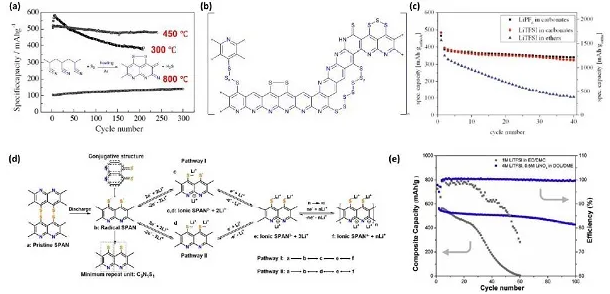
Figure 6. (a) SPAN cycle performance at different temperatures, (b) structure and (c) cycle performance of SPAN, (d) schematic diagram of SPAN lithium storage mechanism, (e) cycle stability of SPAN
Compared with elemental sulfur, SPAN has higher reversible capacity and higher sulfur utilization, as well as relatively stable cycle stability. Although the composite of SPAN with graphene and carbon nanotubes has made great progress in improving electrochemical performance, it still needs to conduct in-depth research on its molecular structure and charge storage mechanism.
1.3 P-type organic sulfide (sulfide) cathode material
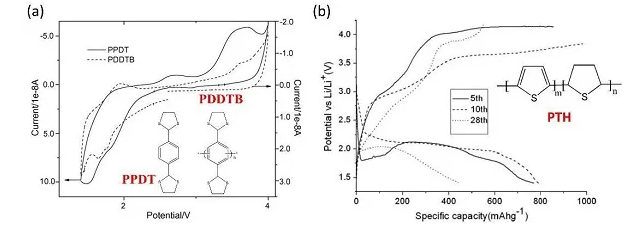
Figure 7. (a) Molecular structure and CV curve of PPDT and PDDTB, (b) Poly-PTH and its charge-discharge curve
Sulfide compounds belong to the p-type organic compounds and exhibit different redox behaviors compared with other organic sulfur compounds. Generally, thioethers are first oxidized to form stable cationic radicals, and then combined with anions in the electrolyte, such as PF6-, ClO4- and TFSI-. Compared with the n-type organic sulfide, the p-type sulfide electrode has a higher redox potential and thus a higher energy density. In addition, the redox process of sulfide is not accompanied by the cleavage and recombination of disulfide bonds, so the positive electrode kinetics is faster than that of disulfide or polysulfide. However, the cycling performance, electrochemical polarization and energy storage mechanism of this type of sulfide cathode material need to be resolved and further studied.
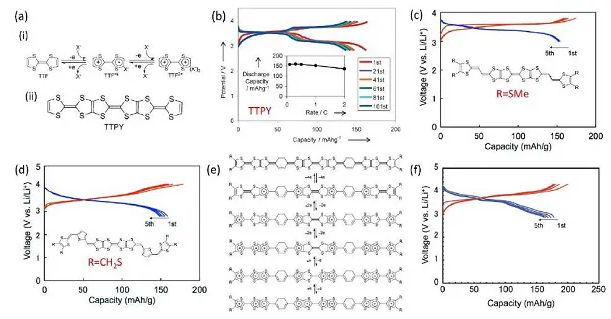
Figure 8. (a) TTF redox process and TTPY molecular structure, (b) TTPY charge and discharge curve, (cd) TTPY-R molecular structure and charge and discharge performance, (e) 10 electron transfer process and TTF electrode (F) Electrochemical performance
Tetrathiafulvalene (TTF) and its derivatives are also p-type organic sulfides, which have metal conductivity and have received extensive attention due to their typical π-electron donor and high-voltage positive electrode characteristics. TTF can occur two consecutive one-electron reversible redox processes. TTPY is insoluble in organic electrolyte, but its discharge products are soluble. By introducing two thiophene rings, a TTPY analog (V-TTPY) was synthesized. This provides a reasonable design strategy for the further development of high energy density organic electrode materials.
1.4 Polysulfide electrode material
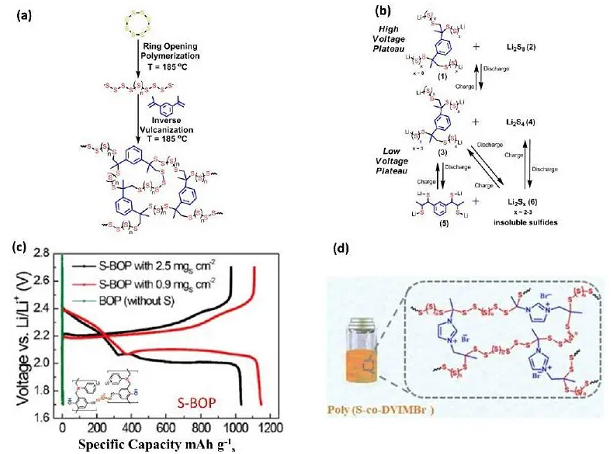
Figure 9. Poly(Sr-DIB) (a) synthesis route and (b) possible lithium insertion/delithiation mechanism, (c) charge and discharge curve of S-BOP, (d) poly(S-co-DVIMBr) Structure diagram
In polysulfides, due to the strong covalent bond between sulfur and the polymer backbone, it can effectively inhibit the dissolution of long-chain polysulfides. The sulfur content can be adjusted by adjusting the quality of the sulfur chains in the polysulfide, and the overall electrochemical performance can also be controlled by introducing favorable organic frameworks or functional groups. Pyun and colleagues systematically studied the lithiation mechanism of the polymer: Under high voltage, short-chain sulfur reacts with Li2S5 to form high-order organic sulfur DIB units; under low voltage, an insoluble mixture of organic sulfur DIB, Li2S3 and Li2S2 is formed. Therefore, the excellent electrochemical performance of poly(Sr-DIB) can be attributed to the dispersion of organic sulfur units in insoluble low-order sulfides. In the new sulfur-rich polymer cathode based on ionic liquids, imidazole cations can be used as chemisorption sites for polysulfides, and the introduction of ionic liquid groups is expected to improve ionic conductivity. The results show that the diffusion kinetics of the copolymer is nearly 10 times higher than that of the copolymer without ionic liquid, indicating that the introduction of cationic functional groups in the organic sulfide polymer provides a new strategy for optimizing the electrochemical performance of organic compounds.
2. Application of organic sulfur compounds in lithium batteries: binders, additives, artificial SEI layers
2.1 Organic sulfides are used as electrolyte additives for Li-S and Li-O2 batteries
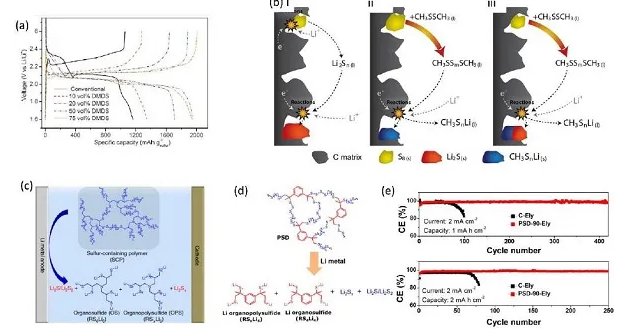
Figure 10. (a) Charge-discharge curve of C/S electrode, (b) schematic diagram of charge-discharge mechanism, (c) schematic diagram of stable inorganic/organic hybrid SEI layer formed by additives on LMA, (d) Li-Cu battery Cycle stability
In the electrolyte containing DMDS additives, the high-voltage discharge platform of the sulfur cathode disappears, and this phenomenon becomes more obvious as the concentration of DMDS increases. More importantly, as the DMDS content in the electrolyte increases, the initial discharge capacity of the Li-S battery also increases. P-type organic sulfides also have good application prospects in Li-O2 batteries, but there is a large overpotential due to the slow electrochemical reaction kinetics. The discharge product Li2O2 usually requires higher decomposition energy, leading to high overpotential and side reactions. Using TTF as a redox medium can promote the decomposition of Li2O2 and provide an additional electron transfer pathway, thereby significantly reducing the overpotential and improving the efficiency of Li-O2 batteries.
2.2 Organic sulfides are used as electrolyte additives or artificial SEI films for protecting lithium negative electrodes
Due to the low Coulomb efficiency of LMA and the potential safety hazards caused by the formation of dendrites, its practical application is hindered. The surface of LMA was modified to improve its electrochemical performance by optimizing electrolyte components, using SEI film-forming additives, and forming artificial SEI. Studies have shown that organic components participate in the formation of SEI and cover the grain boundaries of inorganic components in SEI, thereby significantly improving the flexibility of SEI. This provides a new idea for the development of LMAs inorganic/organic hybrid layer.
2.3 Organic sulfides are used as CEI film-forming additives for inorganic high-voltage cathodes
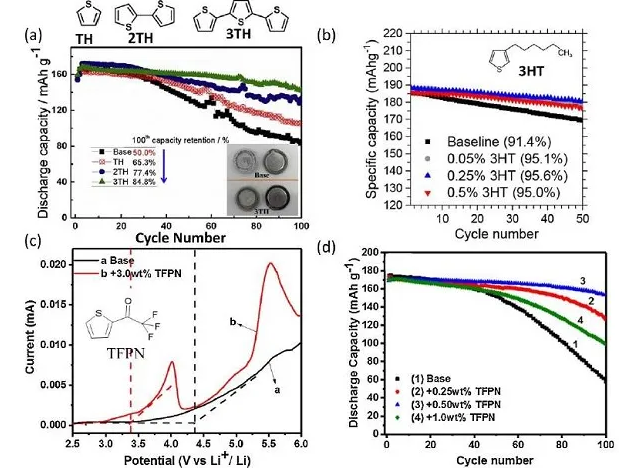
Figure 11. (a) Cycle curve of Li/LiCoO2 battery, (b) Cycle curve of Li/NMC622 battery, (c) LSV curve of LiPF6/EC+DEC, (d) Cycle stability of Li/LiCoO2 battery
Layered transition metal oxides are widely used as cathode materials for commercial lithium ion batteries. A major goal of the next generation of LIBs is to achieve higher operating voltages to further increase the energy density of these batteries. However, the high voltage will cause the battery capacity to decay quickly and the coulombic efficiency in the traditional electrolyte solution. Even in the stable operating voltage window of the electrolyte solution, the side reaction between the electrolyte solution and the positive electrode is difficult to avoid. The formation of a stable CEI layer by electrolyte additives or the modification of the positive electrode surface by doping/coating inorganic or organic components are effective methods to inhibit such side reactions. Although reports of organic sulfides as CEI film-forming additives for high-voltage cathodes are limited, these studies clearly show the potential of organic sulfides to protect high-voltage cathodes.
2.4 Organic sulfides are used as binders or additives for sulfur cathodes
The application of functional binders is of great significance for controlling the dissolution of polysulfides and buffering the volume expansion of sulfur cathodes during cycling. Polyvinylidene fluoride (PVDF) and polyethylene glycol (PEO) cannot inhibit the volume expansion of sulfur cathodes and the shuttle of polysulfides, especially for anodes with higher sulfur content. High conductivity PEDOT: PSS-based conductive polymer used as a binder or coating to improve the electrochemical performance of sulfur cathodes. Studies have shown that organic sulfur polymers can be used as multifunctional binders for sulfur cathodes to improve the sulfur utilization and reaction kinetics of lithium batteries.
image
summary
3. Summary and outlook
This review summarized the influence of different types of organosulfur electrode materials, the structure of organosulfur molecules on electrochemical performance, and effective strategies to improve their cycle stability. Although a lot of work has been done to improve the electrochemical performance of organosulfur electrodes, the large-scale application of organosulfur electrode materials still needs to overcome key problems such as volume expansion, formation of dendrites and dead lithium, and electrolyte consumption. Therefore, this work puts forward several suggestions for further research on organic sulfur electrodes:
3.1 Optimize electrochemical performance
(1) Design more redox active sites and lower molecular weight organic sulfur electrode materials to obtain higher capacity; (2) Use more effective electronic conductive agents to improve the utilization of active materials; (3) Modifications Molecular structure, introducing electron withdrawing groups to increase working potential; (4) Cross-linking of small molecules to form polymers, reducing solubility in electrolytes and improving cycle stability; (5) Introducing highly polar organic groups or using carbon nano Tube, graphene, and foamed carbon synthesize carbon composite material to reduce its solubility in the electrolyte; (6) Combine organic sulfur materials with inorganic nanomaterials to obtain stable cycle performance and rate capability; (7) Use multifunctional binders And modified separating agent to improve cycle stability; (8) Optimize electrolyte.
3.2 Development of advanced characterization technology
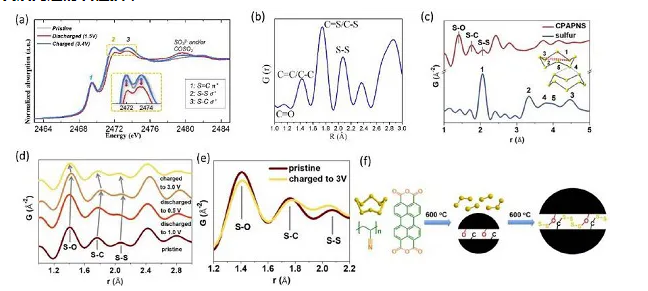
Figure 12. (a) XAS plot and (b) PDF spectrum of TPQD, (c) PDF data of sulfur and CPAPN-S, (d) in-situ charge and discharge of CPAPN-S and (e) PDF charged to 3 V Data, (f) carbon-sulfur compound positive Extremely synthetic route
Advanced characterization techniques (such as XAS and PDF) can not only confirm the structure of organic sulfur cathodes, but also provide direct evidence for the research mechanism. In addition, spatially resolved two-dimensional X-ray fluorescence mapping combined with XAS spectroscopy can intuitively obtain the distribution and valence of sulfur in the electrode and electrolyte.
3.3 Stable lithium metal anode
Electrolyte modification (such as optimizing electrolyte components, introducing SEI film-forming additives, etc.) can adjust the uniform nucleation of lithium, thereby inhibiting the formation of lithium dendrites; using localized high-concentration electrolyte to form a stable SEI layer is also one A promising method. Soluble organic sulfur compounds can also be used as film-forming additives to reduce the reactivity of the electrolyte to LMAs. In addition, the three-dimensional composite LMAs not only suppresses the volume expansion of lithium metal, but also reduces the unevenness of the local current density of the electrode, and makes lithium stripping/plating more uniform.
In general, more efforts are needed in the design of organosulfur electrodes with high sulfur loading and stable structure, in-depth understanding of the relationship between the structural characteristics of organosulfur electrodes and electrochemical performance, and the development of safe and stable new methods. , Which is essential for the development of organic sulfur materials and their use in next-generation rechargeable batteries.
image
Literature link
Review on Organosulfur Materials for Rechargeable Lithium Batteries. (Mater. Horiz., 2020, DOI: 10.1039/D0MH01364A)
Link: https://doi.org/10.1039/D0MH01364A
















