 The latest open access (OA) flagship journal Small Science under WILEY recently published the latest review papers by Professor Jing-Liang Li from Deakin University in Australia and Professor Qu Liangti from Tsinghua University. In recent years, people have paid more and more attention to enhancing the intrinsic reactivity of graphene-based materials by controlling the electrical properties of graphene-based materials without doping with elements. This article reviews the understanding of the intrinsic reactivity of graphene-based materials caused by edges, defects, and free radicals in recent years and their research progress in energy catalysis, biomedicine and other applications. Graphene-based materials, including graphene, graphene oxide (GO), and reduced graphene oxide (rGO), can be used in many fields, such as electronic devices and energy storage, water purification, and environmental remediation (such as adsorption of oil leaks), Light and electrocatalysis for green energy conversion, reinforcement of composite materials, antibacterial drugs and nano-diagnosis and treatment. Some applications make use of the physical (e.g., conductivity, hydrophobicity, and surface area) and chemical (e.g., functional groups) properties of materials, while other applications rely not only on their chemical reactivity, but also on their electrical properties (e.g., catalytic applications) ). Graphene with a perfect honeycomb structure is an inert material. However, in fact, graphene synthesized by different physical and chemical methods contains various defects. These defects include topological defects (edges and holes) and vacancy defects (non-hexagonal rings), and their electronic structure is different from the graphite/graphene base plane. The valence electrons at the dangling groups and defects affect the charge distribution and often lead to the generation of local unpaired electrons (radicals). However, for graphite/graphene, due to limited defects, its inherent activity is much lower than that of traditional metal-based catalysts, so practical applications are limited. In order to produce reactive graphene materials, it is usually doped with some electron-withdrawing elements that can change its electronic structure, such as nitrogen, boron, phosphorus or their combination. Studies have found that in the oxygen reduction reaction (ORR) in fuel cells, the efficiency of doped graphene is equivalent to or even higher than that of platinum-based catalysts. The mechanism of enhancement is that doped atoms can change the charge distribution of adjacent carbon atoms, thereby promoting the adsorption of oxygen and the transfer of charge to the adsorbed oxygen. However, dopant atoms usually require harsh reaction conditions, such as high temperature (eg 300-800°C). In addition, the level of doping must be precisely controlled to obtain the best efficiency. Therefore, people pay more and more attention to enhancing the intrinsic reactivity of graphene materials by controlling the electrical properties of graphene materials without doping elements. In recent years, there have been more and more researches on controlling the inherent reactivity of graphene-based materials by increasing the edges and defects (rather than doping) of graphene-based materials. Recent studies have shown that proper defect engineering can make the catalytic performance of graphene-based catalysts close to or even better than doped catalysts. Defect engineering is a promising method of producing doped and metal-free carbon-based catalysts. It is believed that the reactivity of edges and defects is due to localized electrons and unpaired electrons (radicals). However, the free radical nature of materials has not been characterized and sorted into understanding the mechanisms of different reaction systems.
The latest open access (OA) flagship journal Small Science under WILEY recently published the latest review papers by Professor Jing-Liang Li from Deakin University in Australia and Professor Qu Liangti from Tsinghua University. In recent years, people have paid more and more attention to enhancing the intrinsic reactivity of graphene-based materials by controlling the electrical properties of graphene-based materials without doping with elements. This article reviews the understanding of the intrinsic reactivity of graphene-based materials caused by edges, defects, and free radicals in recent years and their research progress in energy catalysis, biomedicine and other applications. Graphene-based materials, including graphene, graphene oxide (GO), and reduced graphene oxide (rGO), can be used in many fields, such as electronic devices and energy storage, water purification, and environmental remediation (such as adsorption of oil leaks), Light and electrocatalysis for green energy conversion, reinforcement of composite materials, antibacterial drugs and nano-diagnosis and treatment. Some applications make use of the physical (e.g., conductivity, hydrophobicity, and surface area) and chemical (e.g., functional groups) properties of materials, while other applications rely not only on their chemical reactivity, but also on their electrical properties (e.g., catalytic applications) ). Graphene with a perfect honeycomb structure is an inert material. However, in fact, graphene synthesized by different physical and chemical methods contains various defects. These defects include topological defects (edges and holes) and vacancy defects (non-hexagonal rings), and their electronic structure is different from the graphite/graphene base plane. The valence electrons at the dangling groups and defects affect the charge distribution and often lead to the generation of local unpaired electrons (radicals). However, for graphite/graphene, due to limited defects, its inherent activity is much lower than that of traditional metal-based catalysts, so practical applications are limited. In order to produce reactive graphene materials, it is usually doped with some electron-withdrawing elements that can change its electronic structure, such as nitrogen, boron, phosphorus or their combination. Studies have found that in the oxygen reduction reaction (ORR) in fuel cells, the efficiency of doped graphene is equivalent to or even higher than that of platinum-based catalysts. The mechanism of enhancement is that doped atoms can change the charge distribution of adjacent carbon atoms, thereby promoting the adsorption of oxygen and the transfer of charge to the adsorbed oxygen. However, dopant atoms usually require harsh reaction conditions, such as high temperature (eg 300-800°C). In addition, the level of doping must be precisely controlled to obtain the best efficiency. Therefore, people pay more and more attention to enhancing the intrinsic reactivity of graphene materials by controlling the electrical properties of graphene materials without doping elements. In recent years, there have been more and more researches on controlling the inherent reactivity of graphene-based materials by increasing the edges and defects (rather than doping) of graphene-based materials. Recent studies have shown that proper defect engineering can make the catalytic performance of graphene-based catalysts close to or even better than doped catalysts. Defect engineering is a promising method of producing doped and metal-free carbon-based catalysts. It is believed that the reactivity of edges and defects is due to localized electrons and unpaired electrons (radicals). However, the free radical nature of materials has not been characterized and sorted into understanding the mechanisms of different reaction systems.
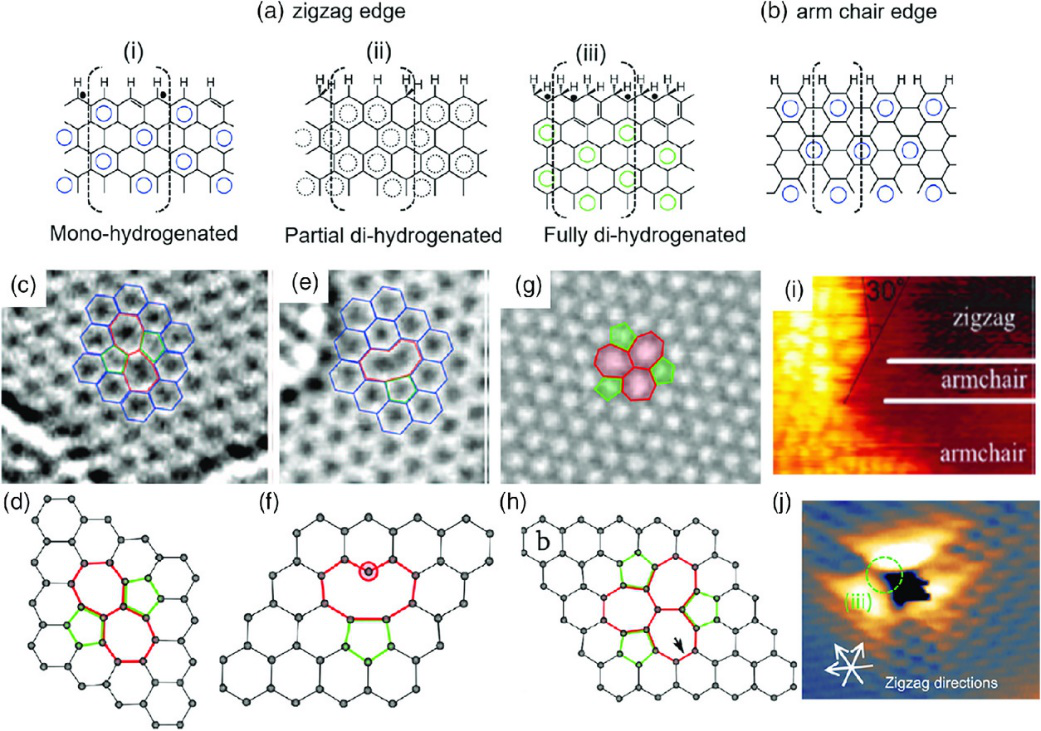
Figure 1 Edge and defect types of graphene-based materials In addition to the defect engineering of graphene, graphene derivative graphene oxide has a very high defect and edge content, and it may also provide a highly efficient catalyst that does not use chemical doping. Strictly speaking, graphene oxide is also doped with oxygen, but as an intermediate for graphene generation, it is generally considered to be different from traditional element doping, which is the introduction of heteroatoms through the heat or plasma treatment of graphene. The synthesis and post-processing conditions determine the complex/multiphase chemistry and structure of graphene oxide (and reduced graphene oxide) and the resulting electrical properties, which may also cause deviations in the effectiveness of certain applications (such as antibacterial applications). Although people have invested a lot of energy in the research of graphene oxide, more research is still needed to understand the correlation between their physical/chemical properties and electronic structure, and how these structures affect their reactivity. It is worth mentioning that in addition to heteroatom doping, defect engineering and oxidation, other methods have been proposed for graphene activation. For example, the use of mild mechanical stress of graphene can enhance its chemical reaction activity to stabilize gold nanoclusters, thereby changing the charge transfer between gold nanoclusters and graphene, reducing the reaction energy barrier of CO catalytic oxidation from about 3.0 eV( No stress) less than 0.2 eV (5% stress). Other substrate engineering methods, such as introducing defects in the Ru(0001) substrate supporting graphene, that is, replacing heteroatoms (such as Au, Cu, Ag, Zn) or single vacancies, have also been used to improve the CO reduction reaction active. It is worth noting that these studies are based on the results of first-principles calculations, and further experiments are needed to support the conclusions.
Figure 2 Application of intrinsic reactivity of graphene-based materials. Graphene materials have various activation methods. Professor Jing-Liang Li from Deakin University in Australia and Professor Qu Liangti from Tsinghua University in this latest paper published in Small Science This article summarizes the understanding of the intrinsic reactivity of graphene-based materials caused by edges, defects, and free radicals in recent years, and its research progress in applications such as catalysis and biomedicine. From the aspects of graphene material edges and defects, the types of defects and their effects on the electronic structure and intrinsic activity are mainly introduced. From the aspect of free radical activation of graphene oxide, the mechanism of chemical bond formation and surface polymerization and the application of materials in photoelectric catalysis, catalytic biosensing, catalytic degradation of environmental pollutants and antibacterial are mainly introduced. Understanding the intrinsic reactivity of graphene materials can eliminate the use of promoters, avoid doping with complex elements, avoid the use of toxic chemicals (such as molecular initiators) for polymerization, and reduce the use of molecular antibiotics that have caused drug resistance. The preparation of low-cost catalysts with low environmental toxicity will further promote the development of carbon-based non-metallic catalysts and their applications in the fields of energy and biomedicine. This article was published online in the latest Small Science (DOI: 10.1002/smsc.202000026).
Source of this article: MaterialsViews






