 In recent years, carbon materials have gradually attracted peoples attention in the fields of electrochemical catalysis and energy storage due to their advantages of good cycle stability, relatively simple process and low cost; green and sustainable biomass carbon materials and their derivatives have gradually become Hot spots in the field of electrocatalysis and energy storage research. The use of nanocellulose to design and develop new and structurally optimized electrocatalysts and electrode materials has high research prospects and commercial value. It not only conforms to the current concept of sustainable development, but also promotes the high-value utilization of nanocellulose. This article mainly reviews the following three aspects: (1) Preparation of nanocellulose; (2) Research status of nanocellulose in the field of electrocatalysis and energy storage; (3) Application of nanocellulose in the field of electrocatalysis and energy storage Outlook.
In recent years, carbon materials have gradually attracted peoples attention in the fields of electrochemical catalysis and energy storage due to their advantages of good cycle stability, relatively simple process and low cost; green and sustainable biomass carbon materials and their derivatives have gradually become Hot spots in the field of electrocatalysis and energy storage research. The use of nanocellulose to design and develop new and structurally optimized electrocatalysts and electrode materials has high research prospects and commercial value. It not only conforms to the current concept of sustainable development, but also promotes the high-value utilization of nanocellulose. This article mainly reviews the following three aspects: (1) Preparation of nanocellulose; (2) Research status of nanocellulose in the field of electrocatalysis and energy storage; (3) Application of nanocellulose in the field of electrocatalysis and energy storage Outlook.
1 Preparation of nano cellulose
According to the different morphological size and preparation method, nanocellulose is generally divided into the following three types (Figure 1): (1) Cellulose nanofibrils (CNF), with a diameter of 5-50 nm and a length of a few microns. (2) Cellulose nanocrystals (CNC), generally 3~55 nm in diameter and 200~500 nm in length. (3) Bacterial cellulose (BC), the diameter is generally 70~140 nm, and the length exceeds 2 μm.
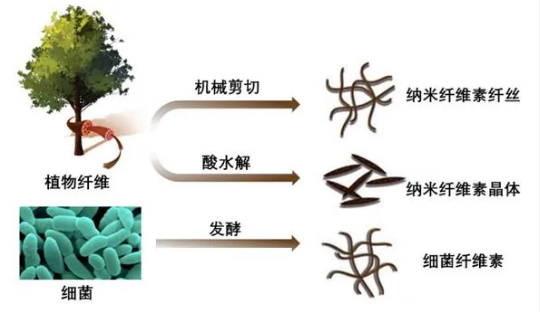
Figure 1 Schematic diagram of nanocellulose preparation
2 Research progress of nanocellulose in the field of electrocatalytic materials
Fuel cells and metal-air batteries are limited by the unfavorable factors of the electrode oxygen reduction reaction (ORR), resulting in certain restrictions on the performance of such batteries. Because of its unique structure, high specific surface area, and chemical modification advantages, nanocellulose can be combined with electroactive materials to prepare new carbon composite materials for ORR electrocatalysis.
With nanocellulose as a carbon-based material, the electronic properties of the carbon material itself can be improved by doping heteroatoms (B, N, S, P, and F, etc.), thereby improving the catalytic activity of the carbon material. Transition metal doping can also improve the electrocatalytic activity of carbon materials, especially nitrogen-containing carbon-supported transition metal materials (M-N-C, M=Fe, Co, Ni, etc.). Nano cellulose is rich in hydroxyl groups, and has a certain adsorption, immobilization and dispersion effect on metal ions. As shown in Figure 2, the addition of CNC improves the dispersibility of metal cobalt nanoparticles, and during the subsequent carbonization process, the CNC releases a large amount of gas when heated, which increases the porosity of the material and exposes more active sites. It can be prepared for ORR and OERs are dual-functional catalysts with excellent catalytic performance.

Figure 2 Schematic diagram of the self-assembly of CoA@CNC composite and its pyrolysis products
3 Research progress of nanocellulose in the field of energy storage materials
Nano cellulose can be converted from non-conductive nano cellulose to conductive carbon material after high temperature carbonization, and it can be used as a carbon electrode of lithium ion battery. The presence of polar-OH groups enhances the hydrophilicity of nanocellulose, while the exposure of hydrophobic-CH groups causes cellulose to have a hydrophobic surface. The presence of hydrophilic and hydrophobic surfaces allows nanocellulose to be used for dispersion. Agent.
Compared with CNC, CNF has a high aspect ratio and a fiber network winding structure, which is easy to form aerogel. Its three-dimensional network structure can provide an efficient path for the migration and diffusion of ions, and provide a large number of active sites for electrostatic adsorption. At the same time, CNF has the characteristics of high conductivity, high specific surface area and low density after carbonization, which is beneficial to the development of electrode materials for flexible and high-strength energy storage devices. Therefore, the composite material prepared with CNF aerogel as the substrate and combined with electroactive materials can be used as an excellent electrode carrier.

Figure 3 Schematic diagram of the preparation process of the three-dimensional porous carbonized CNF/MnOx composite electrode
4 Outlook
The rational design and development of nano-cellulose composite materials will be more concentrated in the future: (1) Research on the regulation and control of nano-cellulose composite materials in the morphology of electrode materials; (2) Precise regulation of the active sites of electrode materials, such as nano-cellulose composite materials. Cellulose is the carbon source carrier to construct MNC single-atom catalyst; (3) Heteroatoms are doped in the pretreatment process of preparing nanocellulose.
Article information: Gao Kun, Sun Jiaojiao, Shen Mengxia, et al. Research progress in electrocatalysis and energy storage materials based on nanocellulose[J]. 2021,40(2): 72-80.







