

MXene is currently one of the most popular two-dimensional materials. However, how to obtain MXene materials that are highly dispersed in organic solvents is still a huge challenge in current research. This article shows Professor Li Huis work on high-concentration MXene organic solvent dispersions. The article introduces in detail the method of preparing high-concentration organic solvents by the surface microenvironment modification method (TMM), and discusses the synthesis mechanism in depth. At the same time, the solution perfectly solves the oxidation problem of MXene. This article provides an efficient preparation path for the future research of MXene, which will help accelerate the research and practical application of MXene materials.
Limited by the hydrophilicity of MXene, how to prepare high-concentration MXene organic solvents has always been an open question. As early as 2017, Professor Yury Gogotsi and others systematically studied the dispersibility of Ti3C2Tx in organic solvents, and the maximum concentration of the final prepared MXene in organic solvents was only less than 0.5 mg/mL. The subsequent development of surface modification methods also requires a large amount of surface modifiers, often requiring surface modifiers of the same order of magnitude as MXene, which greatly hinders the research progress of MXene. The solubility of MXene materials prepared by conventional methods reported in the literature is often less than 0.5 mg/ml. The two existing methods for preparing high-concentration MXene organic solvent dispersions cannot meet the needs of scientific researchers. The solvent replacement method is complicated in operation and low in yield, resulting in waste of materials; surface modification methods often require a large amount of surface modification It is not easy to remove. In composite materials and many water-sensitive applications, the preparation of clean, high-concentration MXene organic solvent dispersions is an urgent scientific problem that hinders the development of this field.
 The results were published online in the top international journal ACS Nano (impact factor 14.588), with the title: High Concentration of Ti3C2Tx MXene in Organic Solvent. Zhang Qingxiao is the first author of this article.
The results were published online in the top international journal ACS Nano (impact factor 14.588), with the title: High Concentration of Ti3C2Tx MXene in Organic Solvent. Zhang Qingxiao is the first author of this article.

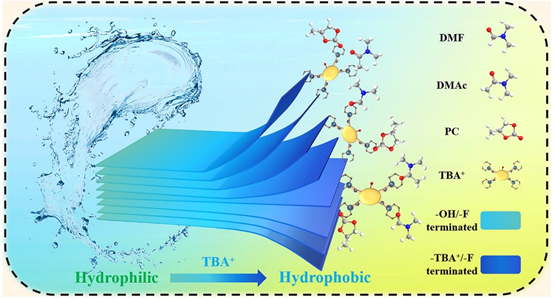 Figure 1. Schematic diagram of the preparation of high-concentration MXene organic solvent materials.
Figure 1. Schematic diagram of the preparation of high-concentration MXene organic solvent materials.
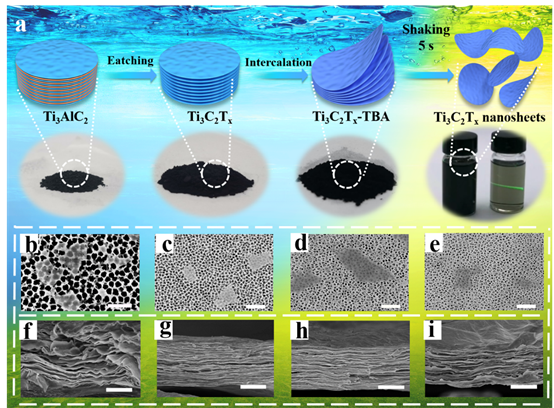 Figure 2. Preparation scheme of high-concentration MXene organic solvent materials and SEM images of Ti3C2Tx nanosheets in different solvents. A more detailed preparation scheme can be found in the experimental part of this article (including the improved MILD preparation scheme)
Figure 2. Preparation scheme of high-concentration MXene organic solvent materials and SEM images of Ti3C2Tx nanosheets in different solvents. A more detailed preparation scheme can be found in the experimental part of this article (including the improved MILD preparation scheme)
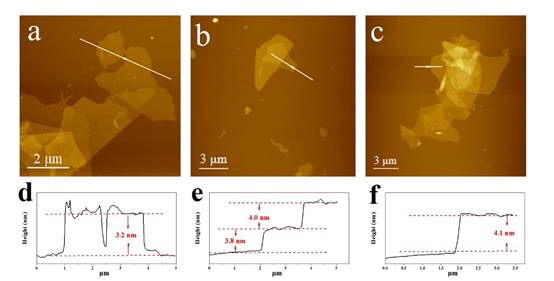 Figure 3. The synthesized two-dimensional Ti3C2Tx AFM images scattered in (a) PC, (b) DMAc, (c) DMF.
Figure 3. The synthesized two-dimensional Ti3C2Tx AFM images scattered in (a) PC, (b) DMAc, (c) DMF.
 Figure 4. Self-supporting membranes prepared from MXene dispersions in different solvents.
Figure 4. Self-supporting membranes prepared from MXene dispersions in different solvents.
 Figure 5. Ultra-high concentration MXene organic solvent dispersion.
Figure 5. Ultra-high concentration MXene organic solvent dispersion.
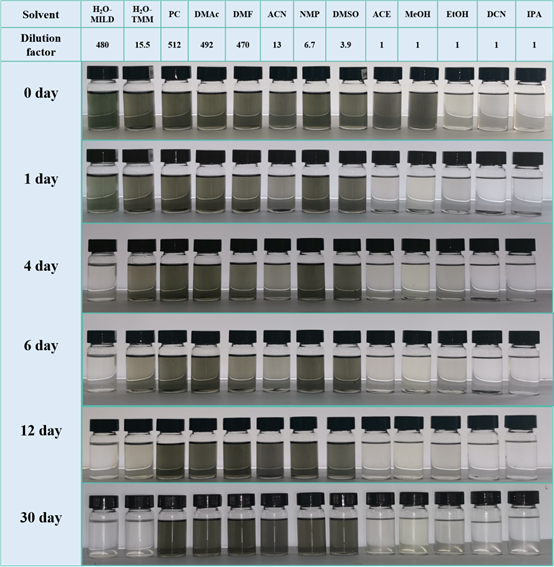 Figure 6. The oxidation stability test of the MXene organic solvent dispersion prepared by the TMM method and the aqueous solution prepared by the MILD method at the same concentration. The article also gives a detailed TMM method and the authors optimized MILD method preparation scheme. This article discusses in detail the oxidative stability of MXene at the same concentration, which is not mentioned in most previous articles. As shown in the figure, it can be clearly and intuitively found that the dispersion of MXene in organic solvents has obvious oxidation resistance.
Figure 6. The oxidation stability test of the MXene organic solvent dispersion prepared by the TMM method and the aqueous solution prepared by the MILD method at the same concentration. The article also gives a detailed TMM method and the authors optimized MILD method preparation scheme. This article discusses in detail the oxidative stability of MXene at the same concentration, which is not mentioned in most previous articles. As shown in the figure, it can be clearly and intuitively found that the dispersion of MXene in organic solvents has obvious oxidation resistance.
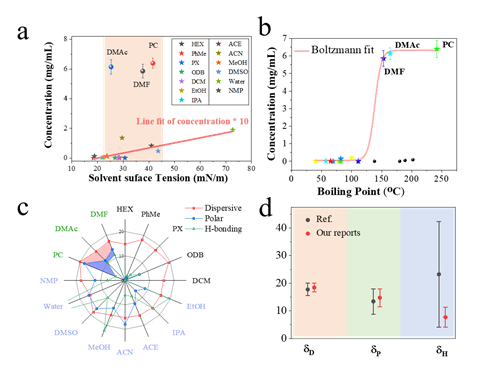 Figure 7. Parameter fitting of MXene and organic solvent.
Figure 7. Parameter fitting of MXene and organic solvent.
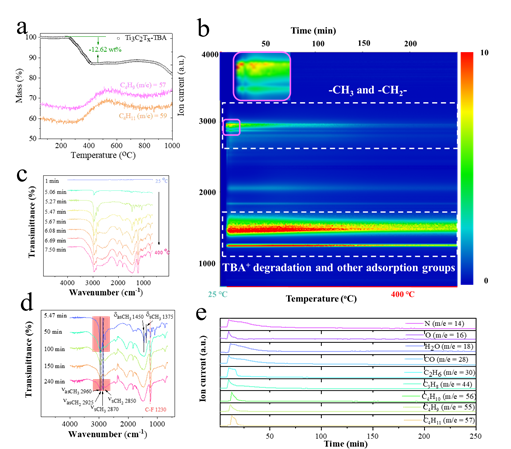 Figure 8. Oxidation stability test of MXene organic solvent prepared by TMM method and aqueous solution prepared by MILD method at the same concentration. The final results in Fig. 7-8 show: 1. The modification of a small amount of TBA+ and the modification of the surface of Ti3C2Tx changed the hydrophilicity and hydrophobicity of MXene. 2. After calculation, the Hansen solubility parameters of Ti3C2Tx are about 18.05 MPa1/2, 14.48 MPa1/2 and 8.76 MPa1/2. This provides a more specific reference for further searching for organic solvent dispersions suitable for MXene.
Figure 8. Oxidation stability test of MXene organic solvent prepared by TMM method and aqueous solution prepared by MILD method at the same concentration. The final results in Fig. 7-8 show: 1. The modification of a small amount of TBA+ and the modification of the surface of Ti3C2Tx changed the hydrophilicity and hydrophobicity of MXene. 2. After calculation, the Hansen solubility parameters of Ti3C2Tx are about 18.05 MPa1/2, 14.48 MPa1/2 and 8.76 MPa1/2. This provides a more specific reference for further searching for organic solvent dispersions suitable for MXene.
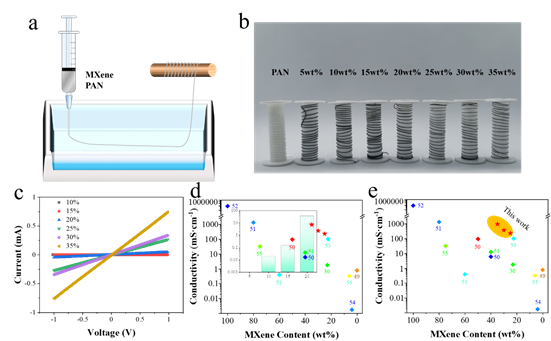 Figure 9. The application potential of high-concentration MXene organic solvent dispersion-wet spinning. In addition, the high-concentration MXene organic solvent dispersion can also be widely used in 3D printing, electrospinning, composite materials and other fields, especially in some water-sensitive fields.
Figure 9. The application potential of high-concentration MXene organic solvent dispersion-wet spinning. In addition, the high-concentration MXene organic solvent dispersion can also be widely used in 3D printing, electrospinning, composite materials and other fields, especially in some water-sensitive fields.
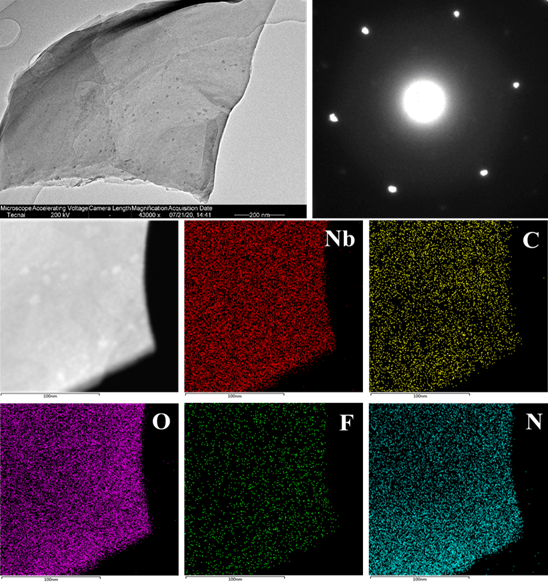 Figure 10. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Nb2CTx.
Figure 10. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Nb2CTx.
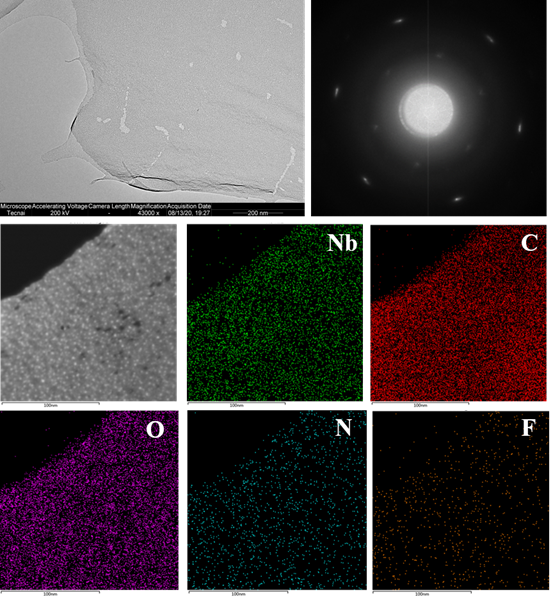 Figure 11. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Nb4C3Tx, Note: Nb4C3Tx oxidizes violently under the strong beam stimulation of high-resolution TEM.
Figure 11. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Nb4C3Tx, Note: Nb4C3Tx oxidizes violently under the strong beam stimulation of high-resolution TEM.
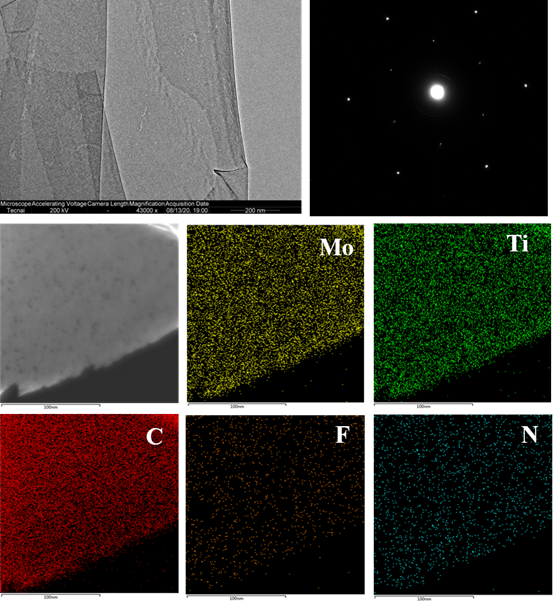 Figure 12. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Mo2Ti2C3Tx, bimetallic MXene can also be perfectly dispersed in organic solvents using TMM method.
The TMM method can be widely applied to the preparation of various MXene materials and is easy to promote.
Figure 12. The expansion of other organic solvent dispersions of MXene prepared by the TMM method. Mo2Ti2C3Tx, bimetallic MXene can also be perfectly dispersed in organic solvents using TMM method.
The TMM method can be widely applied to the preparation of various MXene materials and is easy to promote.
 This study achieved a high degree of dispersion of Ti3C2Tx and other MXene in organic solvents through surface microenvironment modification. The Ti3C2Tx nanosheets obtained by stripping have a clean surface, uniform thickness, and large size. This solution can prepare an organic solvent dispersion with a concentration greater than 90 mg/ml, which greatly improves the dispersion in the MXene organic solvent. Through experimental exploration, it is found that the advantage of the TMM method lies in the intercalation of cations and the change of hydrophilicity and hydrophobicity on the surface of the material, and it also relies on suitable solvent properties to disperse MXene nanosheets. The author systematically studied how to improve the dispersibility of MXene in organic solvents in terms of solvent properties and surface changes of MXene materials. The following conclusions are drawn: 1. The modification of a small amount of TBA+ and the modification of the surface of Ti3C2Tx have changed the hydrophilicity and hydrophobicity of MXene. 2. After calculation, the Hansen solubility parameters of Ti3C2Tx are about 18.05 MPa1/2, 14.48 MPa1/2 and 8.76 MPa1/2. This provides a more specific reference for further searching for organic solvent dispersions suitable for MXene. The stripping yield of the single-layer material in this scheme can reach more than 90%. Using this method, 30 g of multilayer Ti3C2Tx raw materials can be stripped in one pot. The Ti3C2Tx nanosheets obtained by stripping have a clean surface, uniform thickness, and large size. The organic solvent dispersion of Ti3C2TxMXene has excellent oxidation resistance even under aerobic conditions at room temperature. At the same time, the program is simple, efficient, and easy to repeat. This work will bring new opportunities for the research of the MXene material family.
Literature link:
https://pubs.acs.org/doi/10.1021/acsnano.0c10671
This study achieved a high degree of dispersion of Ti3C2Tx and other MXene in organic solvents through surface microenvironment modification. The Ti3C2Tx nanosheets obtained by stripping have a clean surface, uniform thickness, and large size. This solution can prepare an organic solvent dispersion with a concentration greater than 90 mg/ml, which greatly improves the dispersion in the MXene organic solvent. Through experimental exploration, it is found that the advantage of the TMM method lies in the intercalation of cations and the change of hydrophilicity and hydrophobicity on the surface of the material, and it also relies on suitable solvent properties to disperse MXene nanosheets. The author systematically studied how to improve the dispersibility of MXene in organic solvents in terms of solvent properties and surface changes of MXene materials. The following conclusions are drawn: 1. The modification of a small amount of TBA+ and the modification of the surface of Ti3C2Tx have changed the hydrophilicity and hydrophobicity of MXene. 2. After calculation, the Hansen solubility parameters of Ti3C2Tx are about 18.05 MPa1/2, 14.48 MPa1/2 and 8.76 MPa1/2. This provides a more specific reference for further searching for organic solvent dispersions suitable for MXene. The stripping yield of the single-layer material in this scheme can reach more than 90%. Using this method, 30 g of multilayer Ti3C2Tx raw materials can be stripped in one pot. The Ti3C2Tx nanosheets obtained by stripping have a clean surface, uniform thickness, and large size. The organic solvent dispersion of Ti3C2TxMXene has excellent oxidation resistance even under aerobic conditions at room temperature. At the same time, the program is simple, efficient, and easy to repeat. This work will bring new opportunities for the research of the MXene material family.
Literature link:
https://pubs.acs.org/doi/10.1021/acsnano.0c10671



















