I. Overview of the article.
Two-dimensional membrane has attracted wide attention because of its abnormal transport phenomenon. however, the ion separation performance of two-dimensional membrane is lower than that predicted by people. The key step to improve ion selectivity is the need for stable d spacing. Therefore, the author demonstrates a strategy of stabilizing the laminar flow structure of Ti3C2Tx by alginate gel column. After alginate connection, the diameter of the nanochannel is effectively fixed at 7.4 ±0.2atro. therefore, the author shows us a strategy to stabilize the laminar flow structure of Gel by alginate gel column.
The two-dimensional membrane has permeability cutoff and outstanding screening performance for valence cations. The ultra-thin manganese alginate pillared membrane with the same d-spacing has a Na2SO4 content of 100% and high water permeability, which is superior to the state-of-the-art nanofiltration membrane. Based on these findings, the author demonstrates an effective method to adjust the selectivity of ions and provides a new perspective for energy and environment-related applications.
Second, guided reading of picture and text.

Figure 1. Schematic diagram of preparation of two-dimensional layered Ti3C2Tx alginate membrane.
After mixing sodium alginate (SA) solution with Ti3C2Tx colloid solution, SA molecules are firmly and uniformly attached to the surface of nanosheets through hydrogen bonding. Then, the composite SA-Ti3C2Tx nanosheets were assembled into mixed SA-Ti3C2Tx films with layered structure. Finally, the SA-Ti3C2Tx membrane was immersed in the cross-linked solutions of different multivalent Mn ions (Ca2+, Ba2+, Mn2+ and Al3+) to obtain the cross-linked membrane of the hydrogel column in the interlayer spacing.
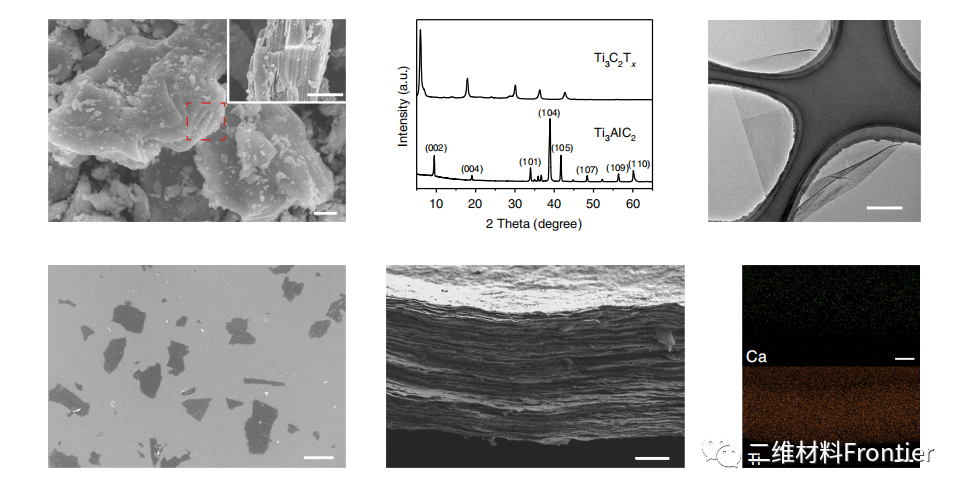
Fig. 2. Characterization of Ti3C2Tx nanowires and calcium-SAT films.
As shown in the figure, the SEM image of the Ti3C2Tx nanosheet deposited on the silicon substrate (1 μ m); the SEM image of the cross section of the Ca-SAT film (scale 1 μ m); and the EDS element map of the Ca-SAT film (scale 1 μ m).

Figure 3. The original interlayer spacing and columnar Ti3C2Tx film.
The XRD pattern and bar chart show the interlayer spacing of the original membrane and the Ca-SAT membrane immersed in pure water or various 0.5molL-1 salt solutions, respectively.

Figure 4.RMFs and T-RMFs photothermal conversion.
Figure A shows the number of sodium ions permeated into the original membrane and Ca-SAT membrane; figure B shows the original and salt membrane permeability of different salts.
III. Summary of the full text.
In this paper, the effect of stable interlayer spacing on the ion transport behavior of Ti3C2Tx membrane connected by calcium-alginate gel was evaluated and compared with that of the original membrane. Under the concentration-driven diffusion of Ca-SAT and the original membrane, the ion concentration increases linearly with time, both univalent and bivalent cations accelerate obviously when diffusing through the cylindrical Ca-SAT membrane, and the increase of PR varies with different values. In particular, the permeability of univalent cations increased nearly three times of that of the original Ti3C2Tx membrane, and still maintained the highest PR.
Extensive studies on nanofluids show that the size repulsion effect of hydrated ions in a very limited environment can not be considered according to the comparison of ion diameter and transport channel size. Although theoretical studies have shown that nanopores with diameters less than 10 should show significant energy barriers, because the hydrate shell around the ions may be deformed or even completely peeled off, so that ions can be entered and transported to the nano-confined space.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.







