I. Overview of the article.
The development of efficient metal or metal compound electrocatalysts under mild conditions has always been a difficult problem in the study of N2 reduction. Here, it is proved that the original two-dimensional MXenes is a promising catalyst for N2 electroreduction, in part because there are multiple active points per unit area. In this paper, a series of 3D, 4d and 5d transition metal M2C (M = Sc, Ti, V, Cr, Mn, Fe, Zr, Nb, Mo, Ta and Hf) are systematically explored and the limit potentials of N2 reduction reaction (NRR) are calculated. The authors found that among the M2CMXenes synthesized today, the free energy barrier (Δ G) produced by 4d4-Mo2C is the lowest 0.46eV. The authors found that two hypothetical MXenes, compared with 4d4-Mo2C, 3d5-Mn2C and 3d6-Fe2C have lower Δ G0.28 and 0.23eV, respectively, so they may be more effective catalysts for NRR.It is found that the N2 capture intensity is a key parameter of the potential limiting step, which is closely related to the arrangement of d electrons on the occupied and empty spin splitting d orbitals. Therefore, the excellent NRR performance of Mn2C and Fe2C can be attributed to the ideal semi-filled 3d5 or 3d6 electronic arrangement. The adsorption of N2 on Mn2C leads to the downward 3D orbit of empty spin of 1 σ electron to Mn. The donor electron weakens the N2 adsorption strength and reduces the energy barrier of the hydrogenation limiting step. The results of this comprehensive study provide guidance for the design of a new type of high efficiency fixed N2 electrocatalyst.
Second, guided reading of picture and text.
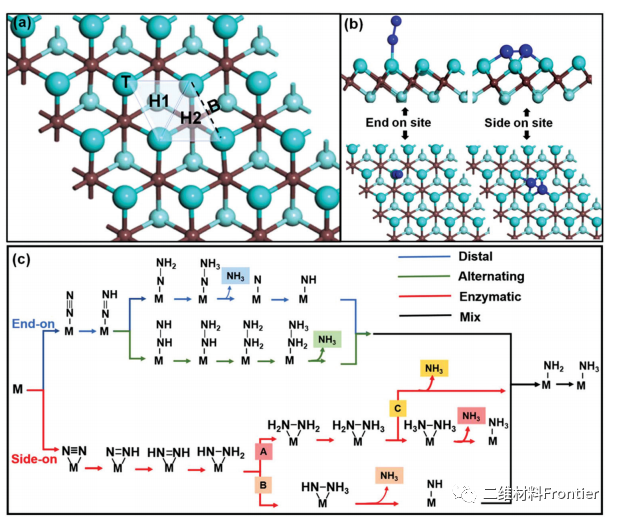
Fig. 1. Different nitrogen adsorption sites of (a) M2C MXenes, including top (T), bridged (B) and hollow (H1 and H2) positions. (b) N2 was adsorbed on the top and hollow sites by end-to-end and side-to-side adsorption, respectively. Blue and brown spheres represent N and C atoms respectively, while large cyan spheres and small cyan spheres represent surface and subsurface metal atoms. Schematic diagram of the distal end, alternation and enzyme mechanism of nitrogen reduction of (c) to NH3.
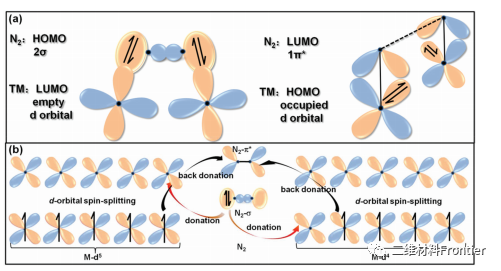
Figure 2. (a) simplifies the diagram of bonding between N2 and M2C MXenes, clarifies the symmetry matching rules and electronic acceptance-donation between HOMO and LUMO. The electronic configurations of (b) d5 and d4 M2C MXenes spin split d orbitals generate spin downward high energy d orbitals and spin upward low energy d orbitals, respectively. Arrows in different directions represent electrons with opposite spins.

Fig. 3. N2-side adsorption on (a) Mn2C MXenes, in which four Mn atoms (Mn1, Mn2, Mn3 and Mn4) bind to N2. Three-dimensional spin-resolved LDOS with spin splitting of Mn atoms in the original Mn2C MXenes of (b). Spin analytic LDOS of 3-D orbitals from Mn1 to Mn4.
III. Summary of the full text.
A series of 3D, 4d and 5d-transition metal M2C (M = Sc, Ti, V, Cr, Mn, Fe, Zr, Nb, Mo, Ta and Hf) MXenes) as potential NRR catalysts were systematically studied. Among the previously synthesized MXenes reported in the literature, 4d4-Mo2C showed the lowest limit potential UL =-0.46V, but the two newly predicted 3d5 (Mn2C) and 3d6 (Fe2C) MXenes both had lower limit potential UL (- 0.28V and-0.23V). With regard to NH3 desorption, it was found that the step-by-step model was more favorable in the enzymatic mechanism. The trapping intensity of N2 is related to the limit potential of NRR, which comes from the arrangement of d electrons on the occupied and empty d orbitals. The predicted excellent catalytic performance of Mn2C can be attributed to the semi-filled three-dimensional electron arrangement. The adsorbed N2 provides 1 σ electrons to the high energy spin downward space three-dimensional orbit of Mn. This electron transfer weakens the adsorption intensity of N2, thus reducing the Δ G in the subsequent potential-limited hydrogenation step. This d-orbital spin splitting rule can be used to guide the design of efficient N2 fixed electrocatalysts in the future.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.






