Background introduction.
Dental caries is a disease caused by biofilm. The interaction between dietary sugar and microorganisms causes acid dissolution of mineralized teeth. If not treated in time, it will lead to systemic complications. This kind of diet plays a key role in destroying the interaction between symbiotic bacteria and conditional pathogenic bacteria on the tooth surface. Excessive sugar intake will lead to a large amount of bacterial extracellular polymers (extracellular polymeric substances, EPS) on the tooth surface, and acidify the biofilm microenvironment associated with dental caries. Pathogenic bacteria, such as Streptococcus mutans, because they can produce a large amount of EPS, metabolize carbohydrates into organic acids, and survive in acidic conditions, can multiply in a large number of oral microenvironments with high sugar, forming biofilms that induce dental caries, and eventually lead to dental caries. Symbiotic bacteria, such as oral streptococcus, can destroy the biofilm of pathogenic bacteria and maintain dental health by producing large amounts of H2O2. However, in the high glucose environment, due to the mass reproduction of pathogenic bacteria and the improvement of metabolic level, it will further aggravate the accumulation of organic acids, resulting in a significant decrease in the sensitivity of oral microenvironment to H2O2, and it is difficult to destroy the biofilm of pathogenic bacteria, which is seriously harmful to dental health.
Therefore, considering that the biofilm inducing dental caries has the characteristics of high sugar and acidic microenvironment, David P. Cormode and Hyun Koo of the University of Pennsylvania designed and synthesized a core-shell composite nanomaterial (Dex-IONP-GOx), which covalently binds glucose oxidase (GOx) on the surface of dextran (Dex) modified iron oxide nanoparticles (IONP). On the one hand, GOx catalyzes the production of H2O2 from a large amount of glucose in the cariogenic biofilm; on the other hand, Dex-IONP with peroxidase-like activity can catalyze H2O2 and locally produce reactive oxygen species (reactive oxygen species,ROS), which can effectively sterilize and degrade EPS matrix. It was found that Dex-IONP-GOx can selectively bind to Streptococcus mutans and kill Streptococcus mutans more effectively than symbiotic bacteria, so it can effectively destroy the biofilm of pathogenic bacteria. Compared with the current standard oral antimicrobial agent (chlorhexidine), Dex-IONP-GOx can more effectively reduce the development of dental caries and will not have harmful effects on the surrounding soft tissue and gastrointestinal microorganisms.
Guided reading of picture and text.
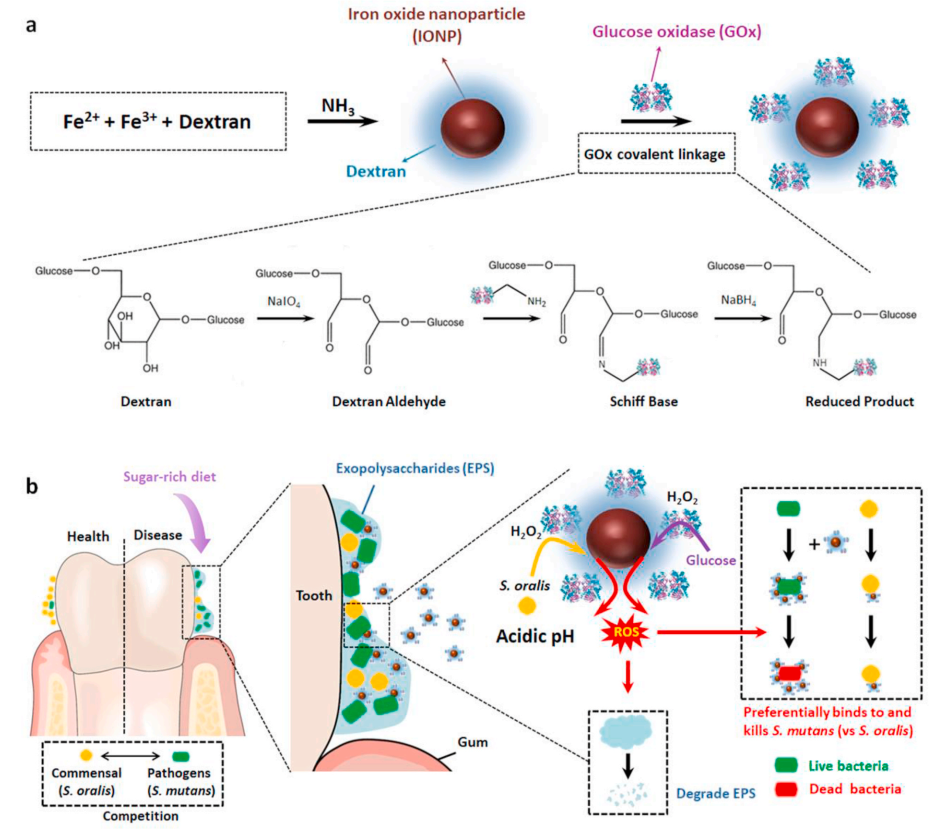
Figure 1.
Schematic diagram of Dex-IONP-GOx targeting pathogenic oral biofilm. (a) the schematic diagram of the preparation process of Dex-IONP-GOx; (b) the schematic diagram of the selective catalysis-therapeutic mechanism of Dex-IONP-GOx to acidic pathogenic biofilm.

Figure 2.
The catalytic activity and anti-biofilm effect of Dex-IONP-GOx. (a, b) transmission electron microscope images of Dex-IONP and Dex-IONP-GOx (TEM); (c) UV-vis spectra of Dex-IONP,GOx and Dex-IONP-GOx; (d) formation of ROS catalyzed by Dex-IONP-GOx with or without glucose at different pH values; (e) relative content of iron in Dex-IONP-GOx and its iron release at pH 4.5. (F) the catalytic activity of Dex-IONP-GOx and its iron ions released at pH 4.5; (g) the antibacterial activity of control group and Dex-IONP,GOx and Dex-IONP-GOx (g) and the dry weight of biofilm after co-incubation with biofilm (h). (I) fluorescence in situ hybridization (FISH) microscope images of control group and Dex-IONP-GOx treated biofilm, Streptococcus mutans were stained by STR405-Cy5 (green), oral streptococci were stained by MIT588-Cy3 (yellow), and (jPowerk) after adding different concentrations of L-ascorbic acid, the antibacterial activity of Dex-IONP-GOx and the dry weight of biofilm after co-incubation with biofilm.

Figure 3.
The bactericidal mechanism of Dex-IONP-GOx. (a) schematic diagram of priority binding of Dex-IONP-GOx to Streptococcus mutans and killing of Streptococcus mutans; (b) binding of Dex-IONP and Dex-IONP-GOx to Streptococcus mutans or Streptococcus mutans; (d) binding of horseradish peroxidase (horseradish peroxidase,HRP) to Streptococcus mutans or Streptococcus mutans; (e) effect of HRP on bacterial survival after the addition of H2O2. (F) the effect of Dex-IONP-GOx on the survival rate of bacteria over time; (g) the image of bacteria co-cultured with Dex-IONP-GOx for different periods of time, the living cells were stained by SYTO 60 (green) and the dead cells were stained by propidium iodide (red); (h) the bacteria treated with Dex-IONP-GOx were detected by ROS, and ROS was stained by HPF (bright blue).
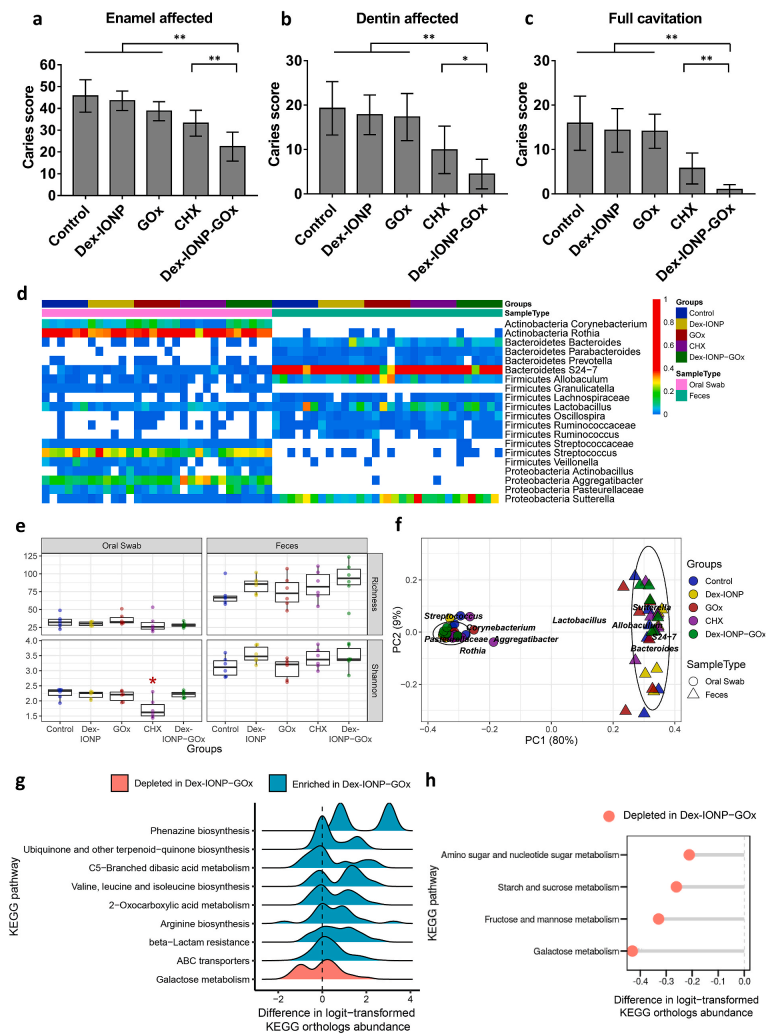
Figure 4.
In vivo therapeutic effect of Dex-IONP-GOx on biofilm-related oral diseases. According to the severity of dental caries, Larson modified Keyes scoring system was used to record dental caries score based on tooth surface; (d) thermographic analysis of major bacterial genera found in all samples; (e) richness and diversity analysis of major bacterial genera; (f) weighted Unifrac principal coordinate analysis (PCoA); and (g) ridge map analysis of predictive pathways in Dex-IONP-GOx.(h) Dex-IONP-GOx involved in the analysis of glucose metabolism-related pathways.
Summary and prospect.
In this work, a composite nanomaterial with double enzyme activity was synthesized based on the microenvironment characteristics of dental caries-related biofilm. Among them, glucose oxidase can catalyze glucose to produce H2O2, while iron oxide nanoparticles with peroxidase-like activity catalyze H2O2 to produce ROS under acidic conditions, destroy the biofilm of pathogenic bacteria, play a targeted killing effect, effectively treat dental caries and maintain oral health.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.







