 [abstract].
[abstract].
Stimulus-responsive supramolecular hydrogels are a new class of waterborne soft materials with a wide range of biological applications. Recently, the team of Professor Masato Ikeda of Gifu University reported a reduction response supramolecular hydrogel constructed from a very simple low molecular weight hydrogel, which is based on a modular molecular design and contains hydrophilic amino sugars and reduction response nitrophenyl.
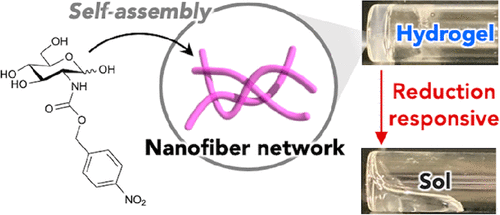
The hydrogel forming ability of glucosamine and galactosamine self-assembly molecules is significantly different. They are differential isomers at position C4. Only glucosamine derivatives can be used as hydrogels. The related paper is published in JACS Au under the title Development of an Amino Sugar-Based Supramolecular Hydrogelator with Reduction Responsiveness.
[guide to the main picture].

Figure 1.
(a) the chemical structure and schematic diagram of GlcN- and GalN-NPmoc self-assembled nanofiber networks and microspheres were studied in this study. (B) the chemical structure of GlcN- and GalN-Fmoc previously reported by Birchall et al. (C) the energy minimized self-assembly structure of GlcN-NPmoc (MMFFs) originates from the single crystal structure of N-acetyl-α-d-glucosamine (α-D-GlcNAc, ACGLUA10), which is shown on the left. There is no possibility of anti-parallel and cross-fingered self-assembly structures, but should not be ruled out. The crystal structure diagrams of α-d-GlcNAc and N-acetyl-α-d-galactosamine are shown on the right.
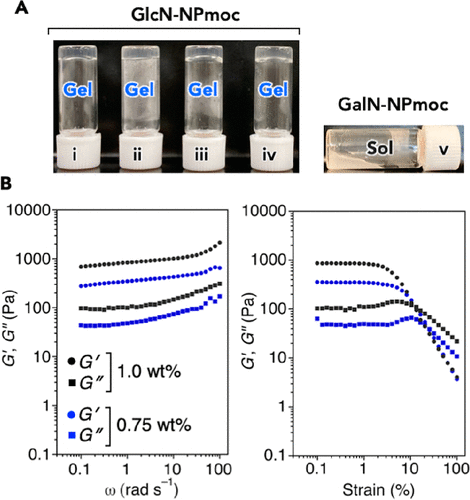
Figure 2.
(a) photographs of GlcN-NPmoc hydrogels prepared with 100mM HEPES-NaOH (pH 7.4): (I) with or without DMSO (5.0vol%), (iii) milli-Q water, (iv) phosphate buffered saline, and (v) GalN-NPmoc dispersions (sol) prepared with 100mM HEPES-NaOH (pH 7.4) containing DMSO (5.0vol%). [GlcN- or GalN-NPmoc] = 0.75wt%. (B) the rheological properties of GlcN-NPmoc hydrogel {[GlcN-NPmoc] = 0.75 and 1.0 wt%,100 mM HEPES-NaOH (pH 7.4) contain DMSO (5.0 vol%): G, storage modulus; G, loss modulus} at 25 °C.
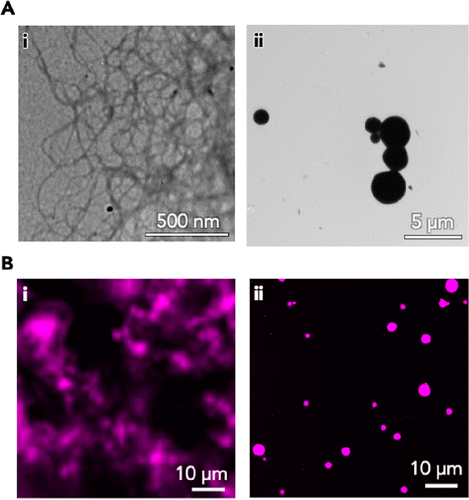
Figure 3.
(I) representative (A) TEM and (B) CLSM images of GlcN-NPmoc hydrogels and (ii) GalN-NPmoc dispersions (sol).

Figure 4.
Representative CLSM images of (A) GlcN-NPmoc hydrogel and (B) GalN-NPmoc dispersion (sol) stained with Nile red (left, magenta) or NBD-H (right, green). (C) histogram analysis of the size of GalN-NPmoc microspheres with or without Nile red staining (evaluated from DIC images). (d) the chemical structure of Nile red and NBD-H and the reaction scheme between GlcN-NPmoc (self-assembly or non-self-assembly) and NBD-H, which should be formed by Hydrazone bond to produce fluorescent molecules.
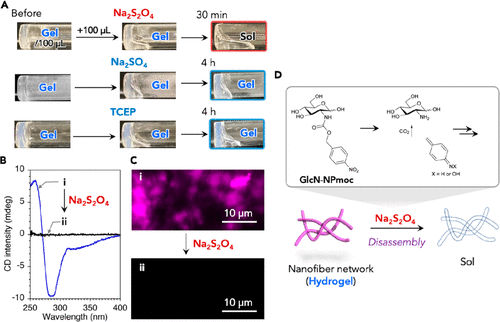
Figure 5.
(a) photos of GlcN-NPmoc hydrogel [1.0 wt% (28 mM), 100 mM HEPES-NaOH (pH 7.4,100 μ L)] before and after the addition of aqueous solution containing Na2S2O4 or Na2S2O4 (20 equiv) or TCEP (1.0 equiv) were used as external stimuli at room temperature. (B) CD spectra and (C) CLSM images of GlcN-NPmoc hydrogels [1.0 wt% (28 mM), 100 mM HEPES-NaOH (pH 7.4)] (I) before and after adding Na2S2O4 (ii) 20 equivalents) at room temperature. (d) the scheme shows that the Na2S2O4 of GlcN-NPmoc hydrogel responds to the transition from gel to sol and the corresponding chemical transformation of GlcN-NPmoc molecule.
[summary].
The team developed a supramolecular hydrogel based on glucosamine, which can spontaneously form supramolecular nanofibers through water self-assembly, resulting in a supramolecular hydrogel with reduction response. This compact but modular molecular design hydrogel, which can be synthesized in one step using two commercially available compounds as a minimum starting material, is one of the simplest low molecular weight supramolecular hydrogels that can provide reductive responsive supramolecular hydrogels.The robustness and simplicity of the modular molecular design can further develop a variety of reduction response waterborne nanomaterials and soft materials with improved properties and functions. For example, the properties and functions of supramolecular microspheres of galactosamine molecules should be further explored; however, this is beyond the scope of the attention of supramolecular hydrogels in this paper. In addition, the biological applications of stimulating responsive supramolecular hydrogels, such as regenerative medicine and cosmetics, may become the target of research in the near future.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.










