[abstract].
Ionic hydrogels are promising candidates for the manufacture of stretchable electronic products, but their applications are seriously limited by the lack of drying and freezing tolerance.Recently, the team of Associate Professor Wu Jin of Sun Yat-sen University reported a simple and general salt penetration strategy for making hydrogels with excellent freeze and drying tolerance, high electrical conductivity and anti-expansion ability. sensitive temperature and strain detection can be performed over a wide temperature range.
The team found that lithium bromide (LiBr) was the most effective hydrogel drying and freezing inhibitor among various salts. Even at-78.5 ¡ãC or ambient air, 50 wt% lithium bromide permeable hydrogels maintain ultra-high tensile properties (625% strain) and electrical conductivity for one year. Density functional theory (DFT) simulations on the molecular scale can be used to understand the important role of LiBr in inhibiting the drying and freezing of hydrogels and reveal the formation of stable Li+-H2O and Br--H2O clusters.It is found that the introduction of LiBr enhances the temperature and strain sensing properties, such as stability and working temperature range.

The multi-functional transparent sensor shows high thermal sensitivity (2.54% / ¡ãC), wide temperature detection range (- 78.5 to 97 ¡ãC), low detection limit (0.1% strain) and low lag and baseline drift in cyclic strain sensing. Due to its high tolerance to a wide temperature range, the hydrogel can maintain strain sensing even at-20 ¡ãC. Use a hydrogel-based epidermal sensor to monitor a variety of physiological signals in real time, such as facial expressions, word pronunciation and knee bending. The related papers are published in Journal of Materials Chemistry C under the title Ultrastable, stretchable, highly conductive and transparent hydrogels enabled by salt-percolation for high-performance temperature and strain sensing.
[guide to the main picture].
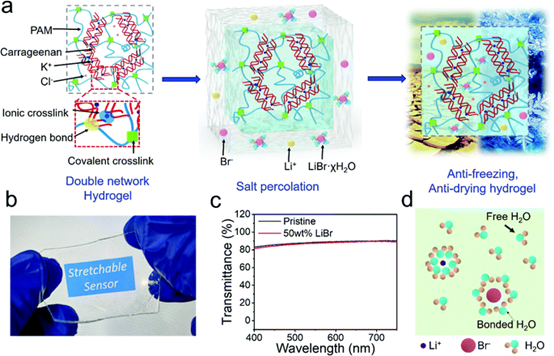
Fig. 1 Salt permeation process and hydrogel characterization.
(a) A schematic diagram showing the preparation of antifreeze and anti-drying hydrogels by salt infiltration. (B) Optical images of 50 wt% lithium bromide permeated hydrogels. The words stretchable sensor on the screen can be clearly observed through the hydrogel. (C) Transmission spectra of 50 wt lithium bromide permeable hydrogels with a thickness of 1.5 mm. (d) cartoons illustrating the interaction between ionized lithium bromide and water molecules. The hydration of dissolved LiBr makes many water molecules fixed around Li+ and Br-, thus enhancing the ability to resist drying and freezing.
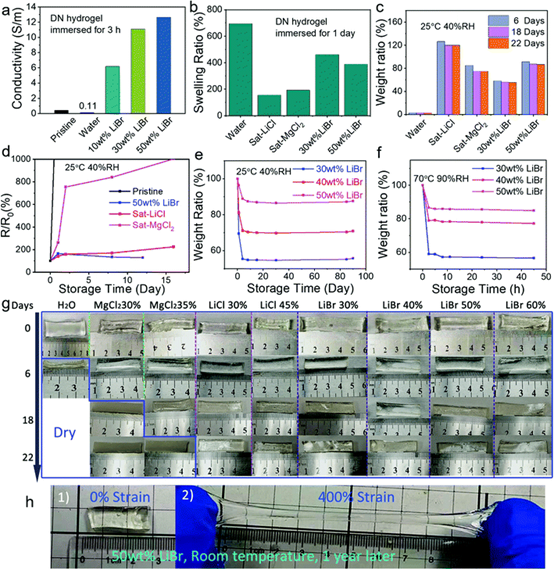
Fig. 2 Anti-drying properties of salt permeable hydrogels.
(a) the electrical conductivity of DN hydrogel before and after soaking in water and lithium bromide aqueous solution for 3 hours. (B) the swelling ratio (W/W0) of DN hydrogel soaked in water, saturated lithium chloride, saturated magnesium chloride and 30 wt% and 50 wt% LiBr aqueous solution for one day. (C) the weight ratio changes of the above hydrogels when placed at 25 ¡ãC and 40% RH for 22 days. (d) the relative resistance changes of the above hydrogels when stored at 25 ¡ãC and 40% RH for about 2 weeks. (e and f) the weight ratio of LiBr hydrogels permeated with different LiBr concentrations changes when exposed to (e) 25 ¡ãC, 40% RH and (f) 70 ¡ãC, 90% RH for different times. (G) the photos show the morphological changes of 10 hydrogel slices after deionized water percolation; 30wt%, 35wt% MgCl2, 30wt% and 45wt% LiCl; were stored at 25 ¡ãC and 40 RH for 22 days, the lithium bromide (h) photos of 30wt%, 40 wt%, 50 wt% and 60 wt%, respectively, showed that 50 wt% LiBr hydrogels were directly exposed to ambient air at (1) 0% strain and (2) 400% strain for one year.
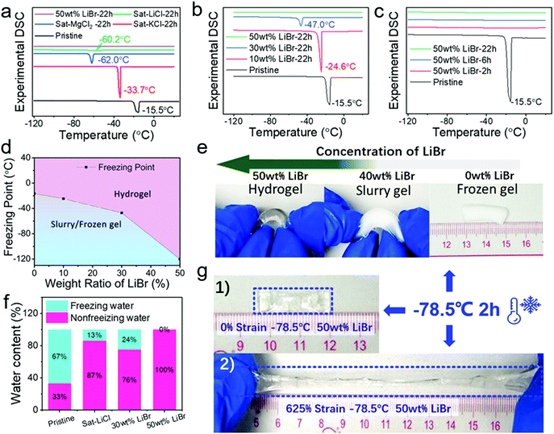
Fig. 3 antifreeze properties of salt permeable hydrogels.
(a) DSC spectra of hydrogels infiltrated for 22 hours with different aqueous solutions (50 wt% LiBr, saturated KCl, LiCl and MgCl2). (B and c) the DSC spectra of hydrogels were permeated with different libr concentrations for 22 hours (b) and 50 wt% libr solution for different times (c). The DSC spectra of the original hydrogels were also recorded for comparison. (d) the phase diagram of lithium bromide permeation hydrogel was constructed by DSC spectroscopy experiment. (e) photographs of unfrozen, slurry and frozen gels were obtained by storing 50 wt%, 40 wt% and 0 wt% lithium bromide hydrogels at-78.5 ¡ãC for 2 hours, respectively. (F) the proportion of frozen and unfrozen water of DN hydrogel before and after immersion in saturated LiCl and 30 wt% and 50 wt% LiBr aqueous solutions. (G) 50 wt% LiBr hydrogel stored at-78.5 ¡ãC for 2 hours under (1) 0% and (2) 625% strain.
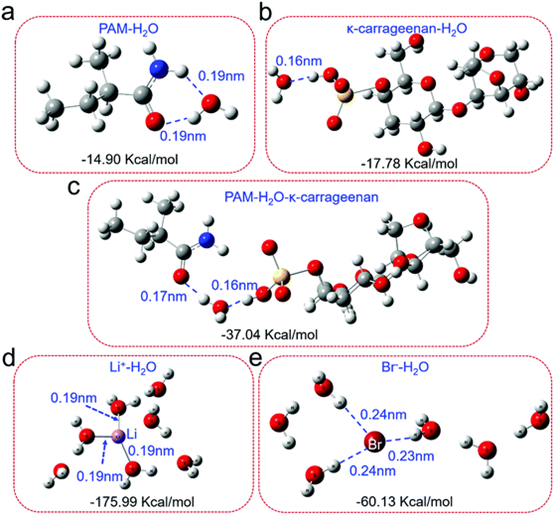
Fig. 4 DFT analysis of the binding of water molecules to various molecules / ions in lithium bromide permeable hydrogels.
The optimized binding configuration of H2O molecule on PAM (a), ¦Ê-carrageenan (b) and PAM/ ¦Ê-carrageenan (c). (d and e) the optimized structure of Li+-H2O and Br--H2O clusters. The corresponding binding energy and intermolecular distance are also shown.
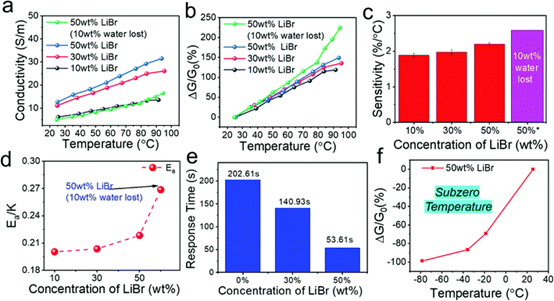
Fig. 5 temperature sensing characteristics of lithium bromide permeable hydrogel.
(a) the conductivity and temperature curves of hydrogels with different libr concentrations. (B) the response of lithium bromide permeation hydrogels with different lithium bromide concentrations (¦¤ G hand G0%) and the temperature curve.(C) the relationship between thermal sensitivity and LiBr concentration. (d) Activation energy of hydrogels with different libr concentrations. (e) the relationship between response time and the concentration of lithium bromide. (F) response of 50 wt% LiBr hydrogel to temperature.

Fig. 6 strain sensing properties of lithium bromide permeable hydrogel.
(a) photos of lithium bromide permeable hydrogels at 0% (top) and 200% tensile strain (bottom). (B) the relationship between the change of relative resistance and strain. Dynamic response to various strains after storage at-20 ¡ãC for 1 day, including 0.1% of small strains (c), 26% of strains (d) and 180% of strains (e). (F and g) dynamic response to repeated loading and unloading 180 cycles for 50% strain. (h) lag of strain sensor.
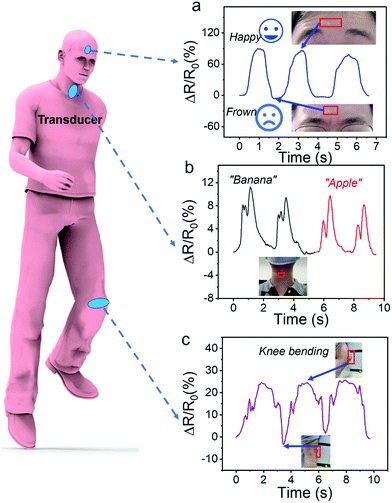
Fig. 7 practical wearable application of hydrogel-based strain sensors.
The response curve to the pronunciation (b) and knee bending (c) of facial expressions frown and happy (a), banana and apple. The photos in the illustration show sensors connected to the corresponding positions of the human body, which are marked with red boxes.
[summary].
The team developed a simple and universal salt penetration strategy to make drying-resistant, freeze-resistant, highly conductive, transparent and expansion-resistant hydrogels for temperature and strain sensing applications. The effects of salts, concentration and soaking time on the conductivity, expansion resistance, dehydration resistance and frost resistance of hydrogels were studied and discussed systematically. Among various salts and concentrations, 50 wt% lithium bromide aqueous solution was found to be the most effective in improving the drying and freezing tolerance of hydrogels. Importantly, the potential mechanism of anti-drying and anti-freezing of lithium bromide permeable hydrogels was revealed by DFT theoretical calculation, and the formation of stable Li+-H2O and Br--H2O clusters was revealed. In addition, the penetration of salt also improves the electrical conductivity and anti-expansion ability of the hydrogel. This is also the first time that LiBr has been introduced into the hydrogel to improve its stability, so that the leachate hydrogel can exhibit extremely high electrical conductivity (12Smmur1), stability and extensibility (up to 625% strain) or ambient air for up to one year, even at-78.5 ¡ãC.
Importantly, the team proposed a multi-functional sensor for temperature and strain based on lithium bromide permeable hydrogel with high sensing performance. The temperature sensor has high thermal sensitivity (2.54% / ¡ãC), wide temperature detection range (- 78.5 to 97 ¡ãC) and short response time (53.6 s). It is found that the concentration of LiBr has a great influence on the sensitivity, response speed and detection range. The tensile strain sensor also shows satisfactory sensitivity (GF = 5.25), low LOD (0.1% strain) and hysteresis, good repeatability, enhanced stability and insensitivity to bending and distortion. Benefiting from the remarkable frost resistance of the hydrogel, the strain sensing ability can be maintained even at-20 ¡ãC, showing a wide working temperature range. When integrated as wearable devices, these features give strain sensors the practical ability to sense a variety of physiological signals, such as facial expressions, word pronunciation, and knee bending.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.











