[abstract].
Recently, inspired by the attraction of metals by magnets, the team of Associate Professor Cai Ming of Tongji University and Professor Cui Wenguo of Shanghai Jiaotong University jointly used the coordination reaction of metal ion ligands to construct bisphosphonate injectable hydrogel microspheres (GelMA-BP-Mg), which can promote cancellous bone remodeling of osteoporotic bone defects by capturing Mg2+. By grafting bisphosphonate (BP) onto GelMA microspheres, GelMA-BP microspheres can produce strong Mg2+ capture ability and sustained release performance through coordination reaction, while sustained release BP has bone targeting properties. In the injectable GelMA-BP-Mg microsphere system, the atomic percentage of captured Mg2+ is 0.6%, and the captured Mg2+ can be effectively released for 18 days.
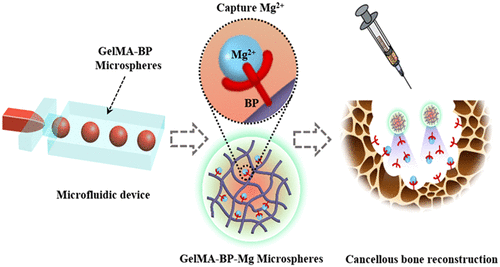
These results show that the composite microspheres can effectively capture Mg2+, which provides a basis for the composite microspheres to activate osteoblasts and endothelial cells and inhibit osteoclasts. The experimental results in vitro and in vivo show that Mg2+ trapping composite microspheres excited by magnets can inhibit osteoclasts by stimulating osteoblasts and endothelial cells, which is beneficial to osteogenesis and angiogenesis, and finally effectively promote cancellous bone regeneration. This study can provide some meaningful ideas for the treatment of osteoporotic bone defects based on metal ions. The related paper is published in ACS Nano under the title Capturing Magnesium Ions via Microfluidic Hydrogel Microspheres for Promoting Cancellous Bone Regeneration.
[picture and text analysis].
Inspired by the natural phenomenon that magnets attract metals, the team chelated Mg2+, on the surface of methacrylonitrile-acylated gelatin (GelMA-BP) microspheres by Schiff base reaction and coordination bonds to construct a capturing Mg2+ microfluidic hydrogel microspheres (GelMA-BP-Mg). Inspired by magnets, the hydrogel microspheres were endowed with active capture of Mg2+, minimally invasive injection, sustained release and bone targeting ability. Thus enhance its activation of osteoblasts and endothelial cells and inhibit osteoclasts, and finally through the integrated multi-purpose microspheres to achieve cancellous bone reconstruction (figure 1).
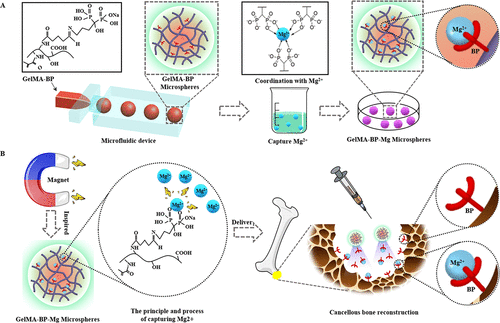
Figure 1. (a) the preparation process of microfluidic GelMA-BP microspheres and the construction of GelMA-BP-Mg microspheres capturing Mg2+. (B) the construction of Mg2+-capturing microfluidic GelMA-BP-Mg microspheres with magnet function and the description of the principle of capturing Mg2+.
Preparation and characterization of Composite Microspheres.
SEM showed that there were BP-Mg nanoparticles with an average particle size of 79 ¡À15nm on the surface of GelMA-BP-Mg microspheres, while there was no such nanostructure on the surfaces of GelMA-BP and GelMA microspheres (Fig. 1A). Energy dispersive X-ray spectroscopy (EDS) showed that GelMA-BP microspheres had uniform P distribution and GelMA-BP-Mg microspheres had uniform magnesium distribution (Fig. 1B). In addition, for each group of composite microsphere samples, EDS showed that there were corresponding P and Mg elements in GelMA-BP and GelMA-BP-Mg observed by SEM (Fig. 1C). 31p NMR spectrum (Fig. 1D) shows that only GelMA-BP and GelMA-BP-Mg have obvious resonance peaks at 17.6ppm. Figure 1E shows the cumulative release curve of BP. It was observed that within 12 days after drug release from GelMA-BP and GelMA-BP-Mg microspheres, the release rate of BP was faster, and then slowed down gradually. 21 days after the release, the release of BP tended to be flat. Figure 1F shows the Mg2+ cumulative release curve of GelMA-BP-Mg microspheres. Figure 1G shows that BP-Mg nanoparticles with a diameter of 77 ¡À12 nm are formed in the system, which is basically the same as the surface nanoparticles of GelMA-BP-Mg microspheres shown by SEM in figure 1G. Figure 1H shows the chemical equations involved in the preparation of composite microspheres that can capture Mg2+.
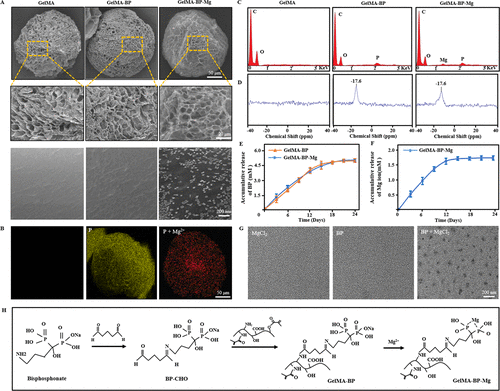
Figure 1. The characteristics of composite microspheres. (a) typical scanning electron microscope images of GelMA, GelMA-BP and GelMA-BP-Mg microspheres at different magnification. (B) the uniform distribution of bisphosphonates on GelMA-BP microspheres and the uniform distribution of bisphosphonates and captured Mg2+ on GelMA-BP-Mg microspheres. (C) Energy spectrum analysis confirmed the existence of corresponding P and Mg elements on the composite microspheres. (d) 31p NMR spectra of three kinds of composite microspheres. The release curves of BP and Mg2+ of (E, F) composite microspheres proved the successful grafting of BP and the effective capture of Mg2+. (G) TEM images of MgCl2 solution, BP solution and the mixture of MgCl2 and BP. (h) the chemical equations involved in the preparation of composite microspheres capable of capturing Mg2+.
Biocompatibility of composite microspheres in vitro.
First, BMSCs and HUVECs cells were cultured in 24-well plates, and then BMSCs and HUVECs cells were co-cultured with GelMA, GelMA-BP and GelMA-BP-Mg microspheres. According to the results of live / dead staining at 1, 3 and 5 days, the composite microspheres were less toxic to co-cultured BMSC and HUVEC (Fig. 2AB, DE). In addition, CCK-8 results further quantitatively verified the cell proliferation activity between each group (Fig. 2C, F). Quantitative statistical analysis showed that the composite microspheres had good biocompatibility. Fig. 3 and panel A-mure of Fig. S5A and B are the results of co-culture of BMSCs and HUVECs cells with GelMA, GelMA-BP and GelMA-BP-Mg microspheres for 2, 5 and 7 days, and the number of cells was quantitatively analyzed. Therefore, these results show that each group of composite microspheres has good biocompatibility.
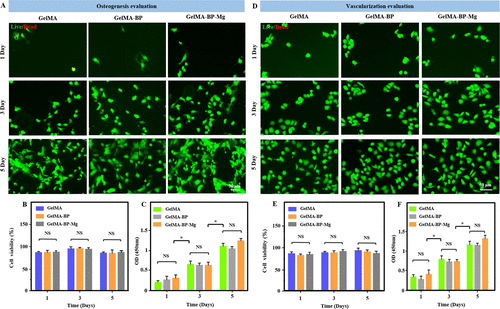
Figure 2. Cell biocompatibility of composite microspheres. (a, D) BMSCs and HUVEC cells were co-cultured with composite microspheres for 1, 3 and 5 days, and analyzed by living / dead staining. Living cells are green and dead cells are red. (B, E) the statistics of the survival rate of the two kinds of cells. (C, F) CCK-8 detected the toxicity of these composite microspheres.
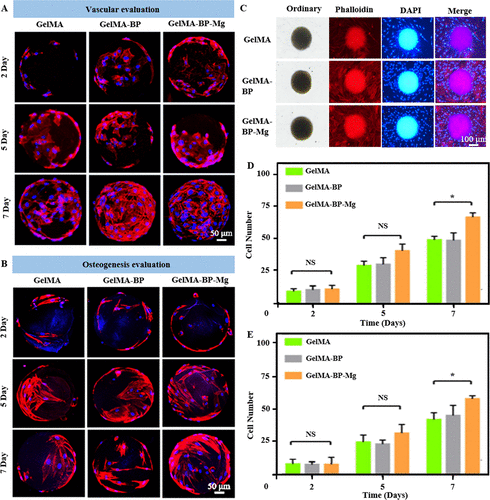
Figure 3. Biocompatibility of composite microspheres.
The proliferation behavior of (A, B) BMSCs and HUVECs cells on composite microspheres was observed for 2, 5 and 7 days. (C) Co-culture of composite microspheres and cells. Red shows the skeleton; blue shows the core.(d) the statistics of the number of cells on the microspheres.
Tube forming experiment.
Figure 4A shows that HUVEC is cultured in the extract of each group of composite microsphere samples at a given point in time. After 3 hours and 6 hours, the tube formation of GelMA-BP-Mg group was better than that of GelMA-BP group and GelMA group, which indicated that the Mg released by composite microspheres had certain angiogenic activity. ImageJ is used to calculate the number of knots and the total length of tubes formed. Therefore, the results showed that these calculated parameters in GelMA-BP-Mg group were higher than those in GelMA-BP and GelMA groups, and were statistically significant (Fig. 4B, C).
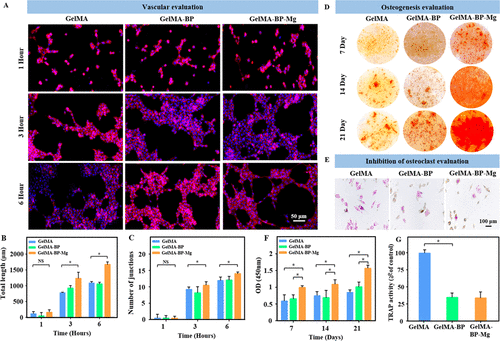
Figure 4. In vitro vascularization, mineralization and osteoclast inhibition of composite microspheres. (a) after 1, 3 and 6 hours of culture, HUVEC cells formed endothelial network. (B, C) the differences of total length (B) and number of nodes (C) between groups were analyzed. (d) Bone marrow mesenchymal stem cells of different microsphere complexes were stained with alizarin red S. (e) TRAP staining microscope pictures of osteoclasts in different microsphere complexes. (F, G) quantitative analysis of alizarin red S staining and TRAP staining in different groups.
Q-PCR and Western blotting evaluation.
After 24 hours of culture, the expression levels of VEGF and e-NOS in GelMA-BP-Mg group were significantly higher than those in GelMA-BP and GelMA groups (Fig. 5A, B, Emurg). Therefore, these results show that the teams composite GelMA-BP-Mg microspheres can effectively release Mg2+ to activate e-NOS and VEGF pathways, thus promoting angiogenesis.
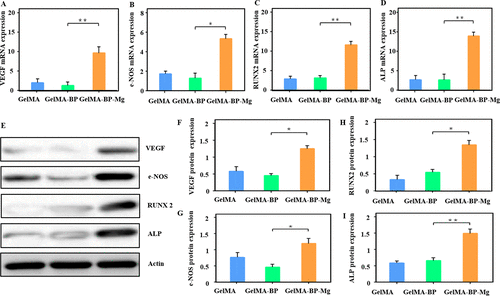
Figure 5. Effects of composite microspheres on the expression of osteogenic and tubular genes in BMSCs and HUVECs cells. (Amurd) Q-PCR showed the expression of related mRNA. These genes include Runx2, ALP, VEGF and e-NOS. (Emuri) Western blotting was used to explore protein expression and make quantitative analysis.
Evaluation of bone regeneration in composite microspheres and Mg2+ capture performance in vivo.
As shown in figure 6A, there was no significant bone regeneration in sham and GelMA groups after 4 and 8 weeks, but the bone quality of GelMA-BP and GelMA-BP-Mg was improved to some extent. Finally the Tb.Th BV/TV and BMD of bones injected with GelMA-BP-Mg were significantly higher than those of GelMA and GelMA-BP groups (figure 6B-H). EDS showed that GelMA-BP microspheres obtained from bone defects had Mg2+ distribution on the surface 1 week and 2 weeks later (Fig. 6i). Therefore, the results show that GelMA-BP microspheres can still effectively capture Mg2+ in vivo.
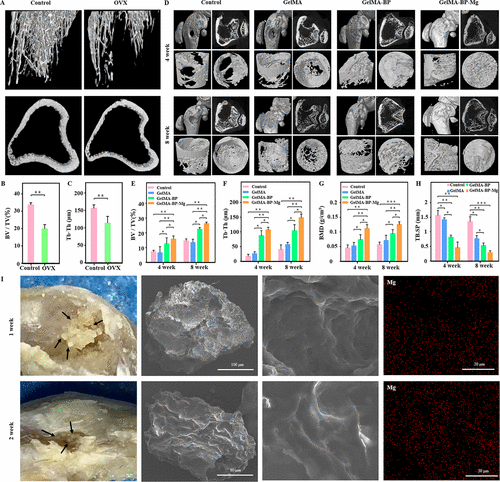
Figure 6. Micro-CT evaluated the effect of injectable composite microspheres on bone regeneration in vivo.The rat model of osteoporosis was successfully established and quantitatively analyzed. Typical microscopic CT images of distal femur of osteoporotic bone defect rats at 4 and 8 weeks. (e) quantitative analysis of micro-CT parameters in different treatment groups: (e) Bv/Tv, (F) Tb.Sp, (G) BMD and (H) Tb.Sp. (I) one week and two weeks after the GelMA-BP microspheres were injected into the bone defect, EDS showed the distribution of Mg2+ on the surface of the microspheres, which proved that the GelMA-BP microspheres could effectively capture Mg2+ in vivo.
Histological evaluation.
HE staining further confirmed the ability of composite microspheres to promote cancellous bone regeneration (Fig. 7A, B). CD31 immunofluorescence was used to test the sections of the fourth week in each group (Fig. 7C, D). The team also performed a histological analysis of the newly formed bone tissue at the bone defect by immunohistochemical staining of type I collagen (figure 7 Eline G), and the results were similar to those of other studies. The results of quantitative analysis showed that the bone defect was still obvious in the control group, but there were more cancellous bone remodeling in the GelMA-BP and GelMA-BP-Mg microsphere injection group. In particular, most of the defects of injecting GelMA-BP-Mg microspheres can be cured. Therefore, the microsphere complex has excellent osteogenic effect, and compared with GelMA-BP and GelMA, GelMA-BP-Mg has stronger cancellous bone regeneration ability.
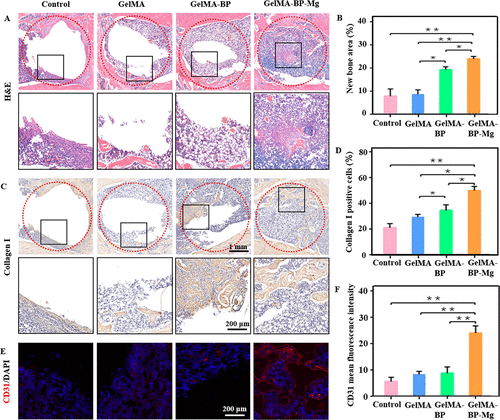
Figure 7. Histological analysis of in vivo treatment of composite microspheres. (a) the representative pictures of HE staining of bone tissue sections in each group after 8 weeks of treatment. (B) the percentage of trabecular bone area in different groups. (C) the representative pictures of femoral tissue sections taken 8 weeks after operation were used for immunohistochemical staining of type I collagen. (d) quantification of type I collagen positive cells. (e, F) Immunofluorescence staining of CD31 expression changes and quantification of protein expression differences in each group.
[summary].
Inspired by the natural phenomenon that magnets attract metals, the team chelated Mg2+ on the surface of BP grafted GelMA-BP microspheres through Schiff base reactivity and coordination bonds. Therefore, a kind of microfluidic GelMA-BP-Mg microspheres capable of capturing Mg2+ with magnet function was constructed, and the hydrogel microspheres were endowed with active capture of Mg2+, minimally invasive injection, sustained release and bone targeting ability, so as to enhance their ability to activate osteoblasts and endothelial cells and inhibit osteoclasts, and finally realize the reconstruction of cancellous bone through integrated multi-purpose microspheres. Obviously, grafting BP onto GelMA microspheres makes GelMA-BP microspheres have strong Mg2+ capture performance and slow release performance. GelMA-BP-Mg composite microspheres showed excellent Mg2+ capture and release, vascularization, osteogenic potential and osteoclast inhibition in vitro. It is further proved that the composite microspheres capturing Mg2+ can promote the regeneration of cancellous bone at the bone defect in rats. The team believes that this magnet-based natural phenomenon can capture Mg2+ composite microspheres and will provide a meaningful concept for clinical osteoporotic bone defect repair.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.












