Early diagnosis of cancer can be made by local treatment measures such as surgery and radiotherapy, which makes it possible to treat primary tumors effectively. Early diagnosis mainly depends on blood biomarkers, however, most biomarkers fall off from the tumor at a very low rate, and are severely diluted by blood circulation and lack of specificity, which hinder the accuracy and sensitivity of early detection. It is worth noting that most human non-communicable diseases (such as cancer, inflammation and thrombosis) affect protease activity, if the detection of protease activity is used as a biomarker of disease, it helps to avoid defects such as lack of sensitivity and specificity, but tools for detecting protease activity usually rely on tedious infrastructure analysis, such as fluorescence, mass spectrometry or magnetic resonance imaging. These techniques require expensive analytical reagents in practice and have poor versatility in different environments.Therefore, in order to achieve the global popularity of precision medicine, it is necessary to develop simple and sensitive diagnostic tools to detect protease activity.
In order to meet the needs of simple and sensitive diagnostic readings, ultra-small gold nanoclusters (AuNCs, < 2 nm) not only have discrete electronic and molecular properties, but also have peroxidase-like activity, which can provide colorimetric measurement of catalytic activity. However, so far, AuNCs has only been used for in vivo applications of fluorescence and X-ray contrast biological imaging. Therefore, it is particularly important to use the catalytic activity of AuNCs to develop a nanosensor that can directly read the disease state by colorimetry.
Based on this, Professor Sangeeta N. Bhatia of Massachusetts Institute of Technology and Professor Molly M. Stevens of Imperial College London constructed a multifunctional protease responsive nanosensor based on biotinylated protease cleavage peptide as template and coupled with neutral avidin (NAv, a biocompatible carrier with high affinity and low specificity for biotin). When injected into colon cancer mice via caudal vein, zinc-dependent matrix protease 9 (MMP9) was specifically cleaved by the tumor site, resulting in the disintegration of the nanosensor assembly, and then AuNCs (2 nm) was filtered into urine through the kidney. Because AuNCs has pseudo-peroxidase activity, it can cause TMB to produce obvious color reaction in the presence of hydrogen peroxide, so that the presence and severity of the tumor can be determined by the naked eye (figure 1).
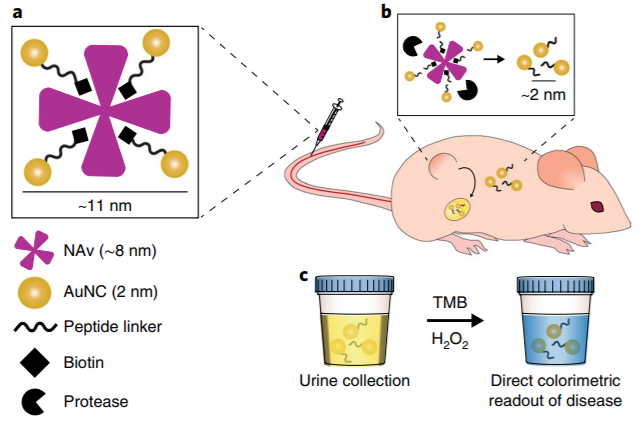
Fig. 1 Design of signal amplification sensing system for nano-catalyst.
Four kinds of polypeptide functionalized AuNCs were synthesized by one-pot method of tripeptide glutathione (GSH, ¦Ã-Glu-Cys-Gly), mercapto functional protease cleavable peptide (P113/P120/P213/P220 (Table 1) and hypochloroauric acid (HAuCl4) (Fig. 2a).Transmission electron microscopy (TEM) showed that the average particle size of GSH-AuNCs was 1.5 ¡À0.4 nm, which was lower than the glomerular filtration limit (~ 5.5 nm), indicating that the nanoclusters could be easily cleared by the kidney (Fig. 2b, c). The pseudo-peroxidase activity of peptide-functionalized TMB was reflected by measuring the absorbance value (A652) of substrate 3-tetramethylbenzidine (TMB) at 652 nm (Fig. 2d). In order to evaluate the sensitivity of the catalytic probe, the authors measured the catalytic activity of peptide functionalized AuNCs in synthetic urine (Fig. 2e). The results showed that the detection limit (LOD) was ~ 2.7pmol (urine 25 ¦Ì l 100 nM AuNCs), and the linear response range was wide, and the dynamic range was more than three orders of magnitude of particle concentration. Because natural horseradish peroxidase is easily degraded by endogenous proteases and hinders its catalytic activity, however, GSH-AuNCs and P220-AuNCs show high stability in physiological environment and maintain high catalytic activity in serum and urine (Fig. 2F).

Fig. 2 AuNCs functionalized by peptides has high catalytic activity.
In order to determine the efficiency of renal particle clearance, the authors determined the content of gold in the urine of peptide-functionalized AuNCs mice by catalytic activity analysis and inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 3a). The results showed that 1 hour after injection, the urine contained 73 ¡À7% peptide-functionalized AuNCs and had high catalytic activity (figure 3b), and the catalytic activity was positively correlated with the results of ICP-MS (figure 3C). Therefore, the colorimetric activity assay of catalytic activity can be used to evaluate the presence of AuNCs in urine simply and sensitively without the need for ICP-MS.
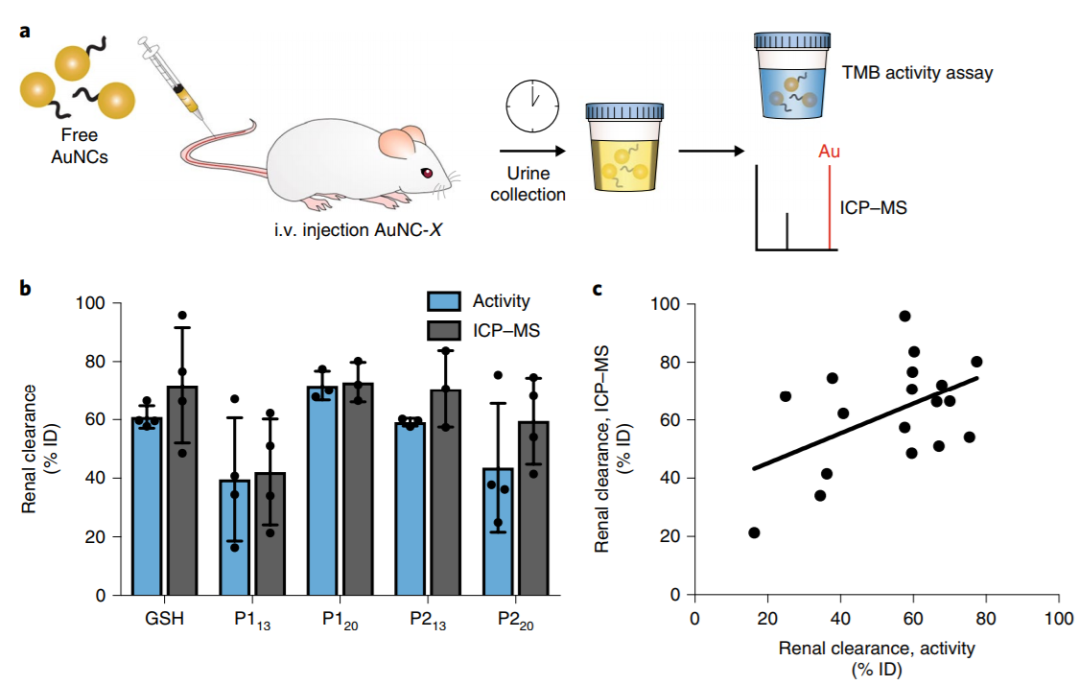
Fig. 3 Peptide-functionalized AuNCs can be cleared by the kidney and expressed in.
Maintain high catalytic activity in urine.
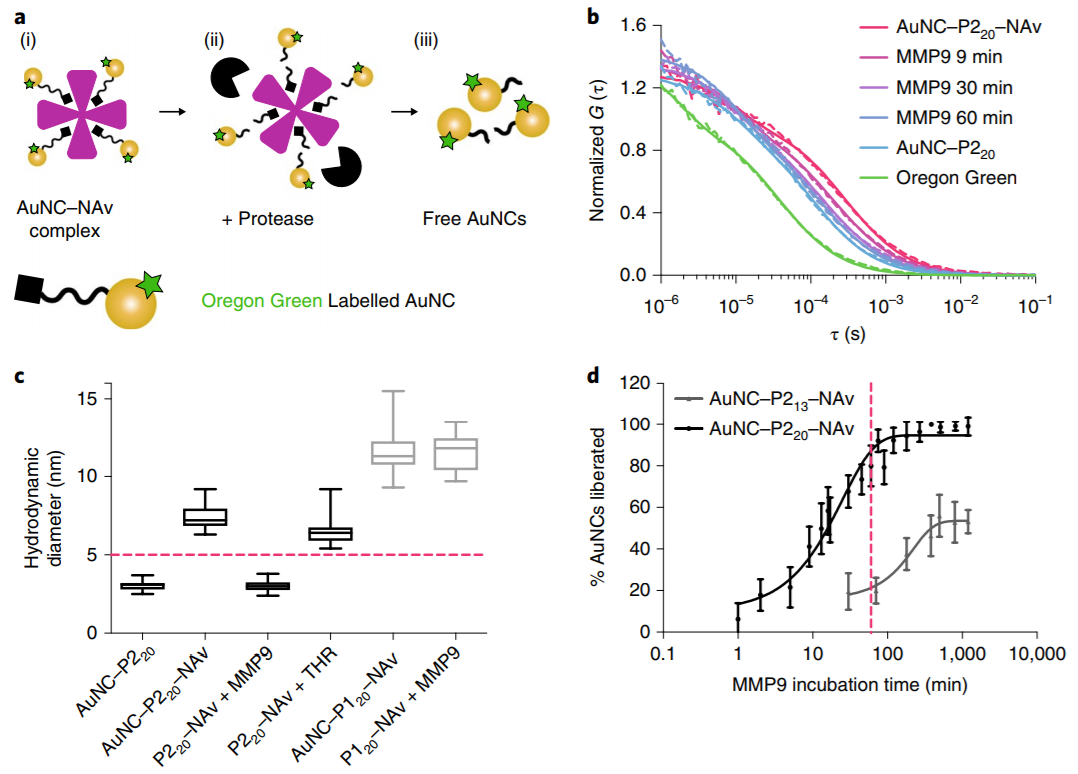
Subsequently, fluorescence correlation spectroscopy (FCS) was used as a single molecule detection method to explore the protein cleavage kinetics of AuNC-NAv complexes (Fig. 4a). When the complex was incubated with MMP9, it was observed that the diffusion coefficient of the complex shifted to the diffusion coefficient of the free fluorescence labeled cluster over time, indicating that the complex had disintegrated (Fig. 4B). In addition, the hydrodynamic size analysis of FCS showed that the AuNC-P220-NAv complex disintegrated completely within 4.5 hours after incubation with MMP9, but when incubated with non-target enzyme-thrombin (THR) for 12 hours, the size of AuNC-P220-NAv complex did not decrease below the glomerular filtration limit (Fig. 4C). The cleavage rate of AuNC-P220-NAv complex by MMP9 (3%) per minute is 40 times higher than that of AuNC-P213-NAv complex (0.08%), which may be attributed to the long P220 peptide chain, which increases the accessibility of the enzyme to the cutting bond. At the same time, about 80% of the AuNC-P220-NAv complexes were incubated and cut by MMP9 within the first hour (Fig. 4d). To sum up, the protease responsive nanosensor designed in this paper is specific to the target enzyme, and the cleavage rate is very fast under the action of the target enzyme.
Fig. 4 in vitro verification of AuNC-NAv complex and protease.
Cleavage process after incubation.
In the in vivo colon cancer detection experiment, both tumor-bearing and healthy mice were injected with MMP9-responsive AuNC-P220-NAv complex through the tail vein (Fig. 5a). Through direct colorimetric reading and initial rate analysis of AuNC catalytic activity cleared from urine (A652 min-1), urine samples of mice were collected 1 hour later for catalytic activity test. it was found that compared with healthy mice, the urine samples of tumor-bearing mice could give the substrate TMB a visible blue color (figure 5b), and the average urine signal of tumor-bearing mice was about 13 times higher than that of healthy mice (figure 5c). In addition, the analysis of the working characteristics of the subjects showed that the area under the curve was 0.91 (Fig. 5d), indicating that the colorimetric test could distinguish the existence of colorectal cancer xenografts with high accuracy. In order to verify that the disintegration of AuNC-NAv complex in vivo is not due to poor chemical stability or non-specific cleavage, the authors injected THR responsive AuNC-P120-NAv complex into tumor-bearing mice and healthy mice. The results showed that compared with healthy mice, the urine samples of tumor-bearing mice did not produce any significant colorimetric signals (Fig. 5e). Therefore, AuNC-NAv nanosensors only respond to the specific protein cleavage activities of diseases in vivo, and can directly read the disease status by colorimetry.

Fig. 5 Protease responsive nanosensor through colorimetric urine.
Read the disease state directly.
In a word, the author developed a method to detect the disease state by colorimetric urine. this method is rapid, simple, sensitive and specific. The designed responsive nanosensor can be used to quickly detect a variety of disease-related proteases, including those related to infectious diseases, so that people can obtain diagnostic information quickly and accurately.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.








