[introduction].
Although the hydrolysis of ester groups is widely used in degradable hydrogels, the breakage in the middle of the main chain will lead to great changes in the mechanical properties of hydrogels. However, the prediction and design of the mechanical properties of hydrogels is a complex task, mainly due to the randomness of the chain-breaking position.
[abstract].
In order to overcome this challenge, Seyoung Kim Assistant Professor, Soo-Hyung Choi Assistant Professor, and Professor Byeong-Su Kim team of Yonsei University proposed a biodegradable ABA triblock poly (ethylene oxide) based hydrogel, which contains acetal pendant with A block, which provides system-adjustable mechanical time characteristics of the hydrogel.In particular, the hydrophobic inner ring tetrahydropyranyl group or the outer ring 1-(cyclohexoxy) ethyl acetal side chain gradually cleaved by acid hydrolysis, resulting in the transformation of gel to sol at room temperature.
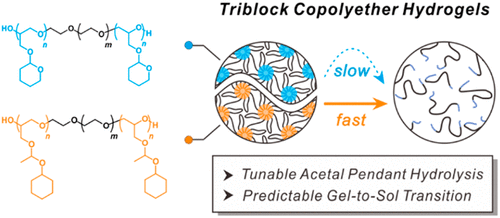
Most importantly, a series of dynamic mechanical analyses combined with non-in-situ NMR spectra show that the hydrolysis rate can be adjusted orthogonally and accurately by changing the chemical structure and hydrophobicity of acetal drapes. This study provides a platform for the development of multi-functional degradable hydrogels in a highly controllable manner. The related paper is published in ACS Macro Letters under the title Hydrolysis-Driven Viscoelastic Transition in Triblock Copolyether Hydrogels with Acetal Pendants.
[guide to the main picture].
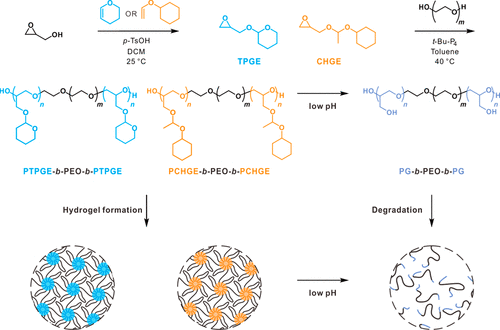
Diagram 1. Synthesis of tetrahydropyran glycidyl ether (TPGE) and 1-(cyclohexoxy) ethyl glycidyl ether (CHGE) monomers, polymerization of triblock copolymers using polyethylene oxide (PEO), and preparation of acidic pH values of hydrogels with controllable degradation.
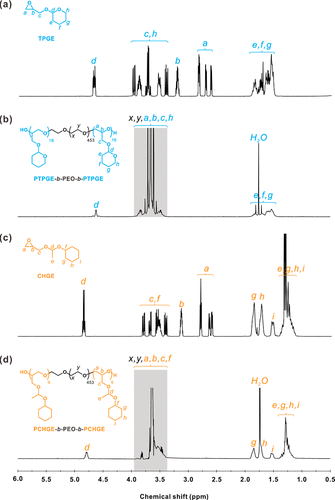
Figure 1. (a) representative 1H NMR spectra of TPGE monomer, (b) PTPGE-b-PEO-b-PTPGE, (c) CHGE monomer and (d) PCHGE-b-PEO-b-PCHGE. In panels (b) and (d), the shaded areas represent the hydrogen of the polyether skeleton. All spectra were collected in CDCl3 at 25 °C.
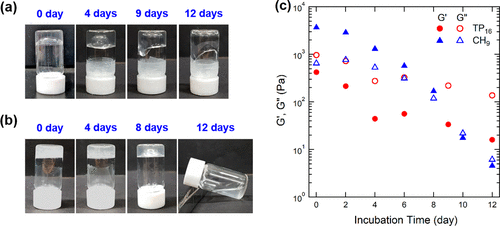
Figure 2. (a, b) photos of (a) TP16 and (b) CH9 hydrogels of pH 5, taken after the incubation target duration of 25 °C. Turn the vial upside down for 20 seconds before taking a snapshot. (C) storage modulus G1 (solid) and loss modulus G2 (open circuit), TP16 (circular) and CH9 (triangular) hydrogels measured at 25 °C at 1Hz frequency and 1% strain at pH 5.
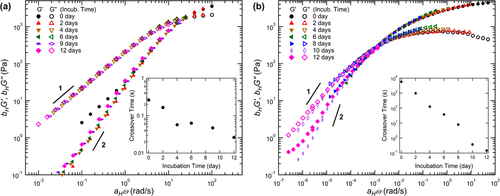
Figure 3. Dynamic modulus G1 (solid) and G2 (hollow) time-hydrolysis superposition. (a) the master curves of TP16 and (b) CH9 hydrogels after pH incubation at 5 °C and 25 °C were obtained by using displacement factors aH and bH. For each curve, the initial sample without incubation (i.e. 0 days) is used as a reference. The illustration shows the crossover time based on the crossover frequencies of G1 and G2.
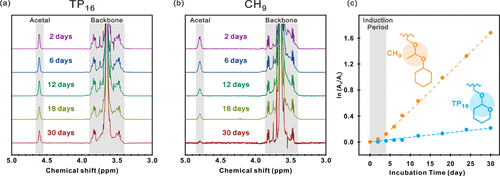
Figure. 4. (a, b) (a) TP16 and (b) the ectopic 1H NMR spectra of CH9 on the acetal bond and polyether skeleton region were obtained after the incubation target duration of pH 5. (C) the reciprocal fraction (A0/At) of the unreacted acetal drape was determined by the 1H NMR peak area of the acetal group at the beginning of incubation (A0) and after the target incubation time (At). For each TP16 (blue) and CH9 (yellow) hydrogels, the data are consistent with the first-order kinetics. The fitting line was shown as a dotted line with a half-life of 87 days and 12 days respectively.
[summary].
Combined with the kinetic analysis of acetal hydrolysis, the team carried out rheological analysis of ABA triblock copolyether hydrogel containing pyrolyzable acetal drape. Due to the cleavage of hydrophobic side groups, the hydrophobicity of A-terminal blocks decreased continuously, and the hydrogel showed a decrease in relaxation time, resulting in a gel-sol transition at room temperature. This rheological change is successfully described in the framework of time-hydrolysis superposition practice, because with the progress of hydrolysis, the terminal block gradually becomes hydrophilic. In addition, compared with CH9 hydrogels, TP16 hydrogels show slower gel-sol transition kinetics during hydrolysis, which is due to the restriction of cyclic acetal side groups on inner ring cleavage in TP16. The observation results show that the hydrophobicity of acetal side group and the structure of outer ring / inner ring control the elastic modulus and softening kinetics of hydrogel. In general, the results show that, different from the widely used hydrogels, amphiphilic triblock copolymers with acetal side groups can promote the systematic tunability of viscoelastic transition in hydrogels.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.









