
Research Background
Facing the growing demand for portable electronic devices, electric vehicles and renewable energy, there is an urgent need to develop energy storage devices with low cost, high energy density and long service life. Technical analysis shows that when the surface energy density of Li-S battery reaches 4 mAh cm-2, it can compete with the latest Li-ion battery technology to reflect its advantages of high energy density. However, thick sulfur electrodes under high load are faced with Challenges of volume expansion, slow kinetics and poor cycle performance. This work is based on the MXene material, which has a strong adsorption effect on polysulfides and has high conductivity. From the aspects of positive electrode structure design and separator modification, a comprehensive design that can accommodate a large amount of sulfur, effectively trap polysulfides, and maintain high-efficiency ions in the circulation And the structure of electronically conductive materials is designed to build a Li-S battery that can cycle stably under high sulfur loading.
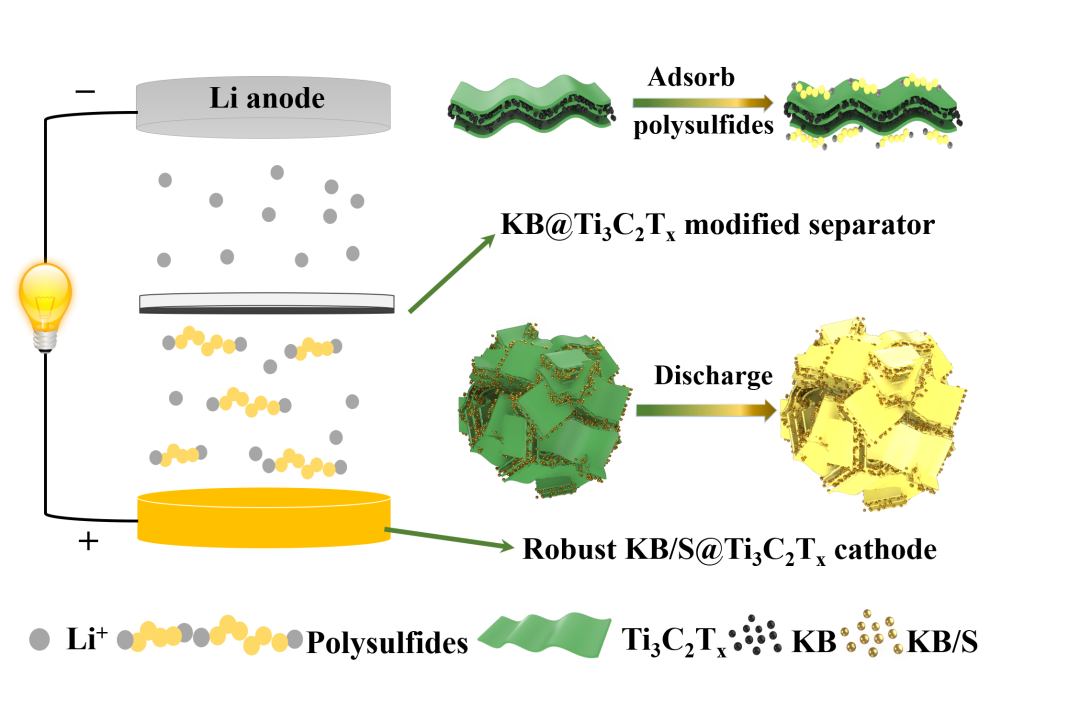
Comprehensive Design of the High Sulfur-Loading Li-S Battery Based on MXene Nanosheets
Shouzheng Zhang1,3‡, Ning Zhong2‡, Xing Zhou2, Mingjie Zhang2, Xiangping Huang3, Xuelin Yang1*, Ruijin Meng2, Xiao Liang2*
Nano‑Micro Lett. (2020) 12:112
https://doi.org/10.1007/s40820-020-00449-7
Highlights of this article
1. Electrostatically self-assemble the negatively charged MXene nanosheets and positively charged Ketjen black/sulfur (KB/S) or KB to construct a composite material with an interwoven structure.
2. The designed KB/S@Ti3C2Tx structural framework allows high sulfur loads and adapts to its corresponding volume changes, maintaining good ion and electron transport capabilities while maintaining structural integrity.
3. The KB@Ti3C2Tx intermediate layer further suppresses the polysulfide escaping from the sulfur cathode. In addition, its areal density is only 0.28 mg cm-2 and its thickness is only 3 μm, which has minimal impact on battery energy density.
brief introduction
The Yang Xuelin team of the School of Materials and Chemical Engineering of Three Gorges University and the Liang Xiao team of the School of Chemistry and Chemical Engineering of Hunan University worked together. The MXene nanosheets are wrapped, and the KB/S@Ti3C2Tx composite cathode material and KB@Ti3C2Tx composite material with an interwoven structure are prepared by electrostatic self-assembly to construct a robust sulfur cathode and an intermediate layer modified separator, respectively. This comprehensive design can maintain the stability of the thick electrode under high sulfur loading, significantly inhibit the escape of polysulfides from the electrode and avoid the collapse of the electrode structure, and the modified diaphragm further hinders the shuttle effect of polysulfides, thus showing Excellent electrochemical performance.
Here, we have conducted a comprehensive design of electrode materials and battery structure based on MXene nanosheets, aiming to achieve the stable cycle performance of high-sulfur surface-loaded Li-S batteries. The inherently negatively charged MXene nanosheets were assembled on positively charged Ketjen black/sulfur (KB/S) particles to construct an interwoven KB/S@Ti3C2Tx composite. Ketjen Black improves the conductivity of sulfur, and Ti3C2Tx ensures the physical/chemical adsorption of soluble polysulfides and further improves the conductivity. More importantly, the self-assembled secondary particle structure significantly maintains the structural stability of the sulfur electrode under volume changes during charging and discharging. KB@Ti3C2Tx prepared by a similar self-assembly method is coated on a commercial separator to further hinder polysulfides that may escape from the cathode. The area load of the KB@Ti3C2Tx intermediate layer is only 0.28 mg cm-2 and the thickness is 3 μm, which has little effect on the volume and quality of the thick electrode, so it hardly affects the energy density of the battery. By combining the robust KB/S@Ti3C2Tx electrode and the effective KB@Ti3C2Tx modified separator, we have obtained a Li-S battery that is stable in circulation with a relatively small amount of electrolyte, and has a relatively high sulfur surface load (5.6 mg cm-2) and higher surface capacity (6.4 mAh cm-2).
Graphic guide
Preparation of I KB/S@Ti3C2Tx electrode material and KB@Ti3C2Tx coating material
Figure 1a is a schematic diagram of the preparation process of KB/S@Ti3C2Tx electrode material. The LiF/HCl mixed solution was selected as the etchant to prepare single-layer Ti3C2Tx nanosheets. The sulfur-loaded KB/S material is modified with PEI to make it positively charged. Through the interaction of positive and negative charges, the inherently negatively charged Ti3C2Tx nanosheets and KB/S self-assemble to form secondary particles. The composite material is KB/S /S is the core, and Ti3C2Tx nanosheets are wrapped layer by layer and have an interwoven structure. Figure 1b characterizes the ZETA potential of each material and proves that the electrostatic self-assembly is successfully carried out. The positively charged material KB/S-PEI and the negatively charged Ti3C2Tx nanosheets have excellent dispersibility in water. When the two are mixed, they will self-assemble and settle due to electrostatic action. The preparation method of KB@Ti3C2Tx coating material is similar. Figures 1d, e and Figures 1f, g are SEM images of KB/S and KB/S@Ti3C2Tx, respectively. It can be clearly seen that Ti3C2Tx nanosheets are uniformly assembled on KB/S to form secondary particles of about 10 μm.
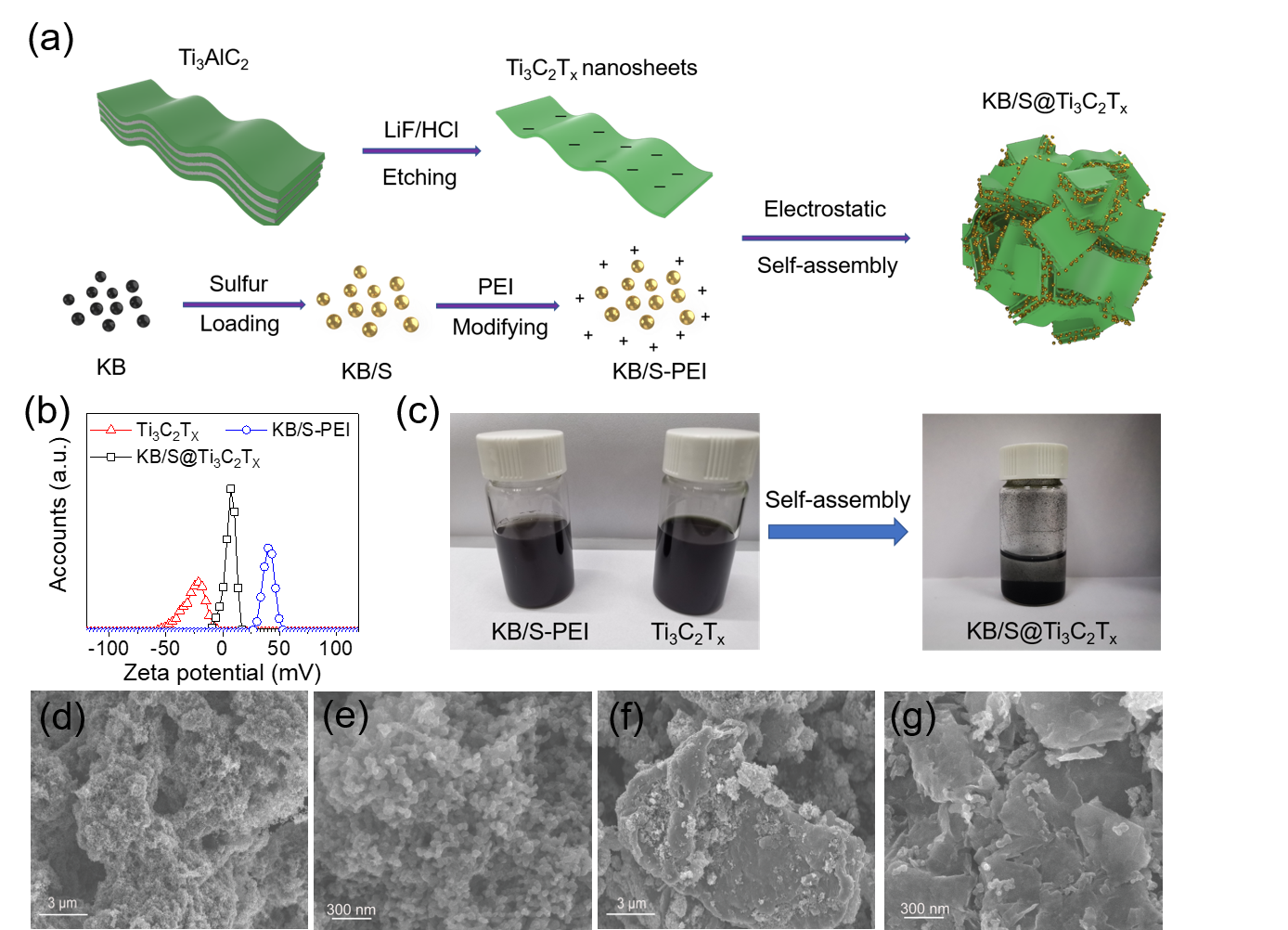
Figure 1. (a) Schematic diagram of the manufacture of KB/S@Ti3C2Tx composite material. (b) The zeta potential of Ti3C2Tx nanosheets, KB/S-PEI and KB/S@Ti3C2Tx. (c) Digital photos of KB/S-PEI, Ti3C2Tx and KB/S@Ti3C2Tx aqueous suspensions. SEM images of KB/S(d,e) and KB/S@Ti3C2Tx(f,h).
II KB/S@Ti3C2Tx Electrode Material Electrochemical Performance and Anti-Volume Change Ability
We systematically evaluated the changes in the electrode structure of KB/S@Ti3C2Tx and KB/S electrodes during cycling through SEM characterization. As shown in Figure 2a, c, the electrode materials of the KB/S@Ti3C2Tx electrode and the KB/S electrode are tightly interconnected when they are not cycled, but after 10 cycles, the upper surface of the KB/S electrode is obviously damaged And cracks (Figure 2d), while the KB/S@Ti3C2Tx electrode did not detect obvious damage to the electrode (Figure 2b). The cross-sectional SEM image shows more detailed information about the stability of the electrode structure. Before the cycle, the thickness of the two electrodes is the same. The tightly connected particles of the KB/S@Ti3C2Tx electrode expanded slightly by about 18% (Figure 2e, f), while the KB/S electrode collapsed during charging, leaving cracks and holes in the electrode (Figure 2g, h). We hypothesize that the dissolution of polysulfides is the cause of the collapse of the KB/S electrode, leading to loss of active material and leaving holes in the electrode. This difference shows that the KB/S@Ti3C2Tx composite material has the ability to keep the active material in the electrode, and the structure of the secondary particles can adapt to the volume change during the cycle, thereby effectively maintaining the integrity of the electrode.

Figure 2. Comparison of two sulfur electrodes through SEM images (a) KB/S@Ti3C2Tx electrode without cycling, (b) KB/S@Ti3C2Tx electrode after 10 cycles, (c) KB/S electrode without cycling, (d) SEM image of KB/S electrode after 10 cycles. Cross-sectional SEM images of KB/S@Ti3C2Tx electrode before (e) and after 10 cycles (f), (g) uncycled KB/S electrode and (h) KB/S electrode after 10 cycles Cross-sectional SEM image.
Figure 3a is the voltage curve of the KB/S@Ti3C2Tx electrode at various magnifications in the range of 0.2 C to 2 C. Even at high magnification, it can still show an obvious dual-voltage platform. Figure 3b shows the excellent rate performance of KB/S@Ti3C2Tx. Figure 3c shows that the KB/S@Ti3C2Tx electrode has better electrochemical performance than the KB/S electrode. At a rate of 0.2 C, after 100 cycles, the discharge capacity of the KB/S@Ti3C2Tx electrode is 812 mAh/g, while the discharge capacity of the KB/S electrode is only 603 mAh/g. Figure 3d shows the long cycle of KB/S@Ti3C2Tx at 0.5 C, showing its stability in 400 cycles. Figure 3e shows the electrochemical performance of the KB/S@Ti3C2Tx electrode when the sulfur load is 4.5 mg cm-2. In the first two cycles, the battery was activated at 0.05C, and then cycled at 0.2C for a long time. The discharge capacities at 0.05 and 0.2 C are 920 and 655 mAh/g, respectively. After 100 cycles, the capacity remains 551 mAh/g. In sharp contrast, the KB/S battery can only show a lower capacity and decay rapidly even under a lower sulfur surface load (2.6 mg cm-2). Figure 3f shows the EIS test of the KB/S and KB/S@Ti3C2Tx electrodes. The lower charge transfer resistance indicates that the sulfur redox kinetics in the KB/S@Ti3C2Tx electrode has been improved.
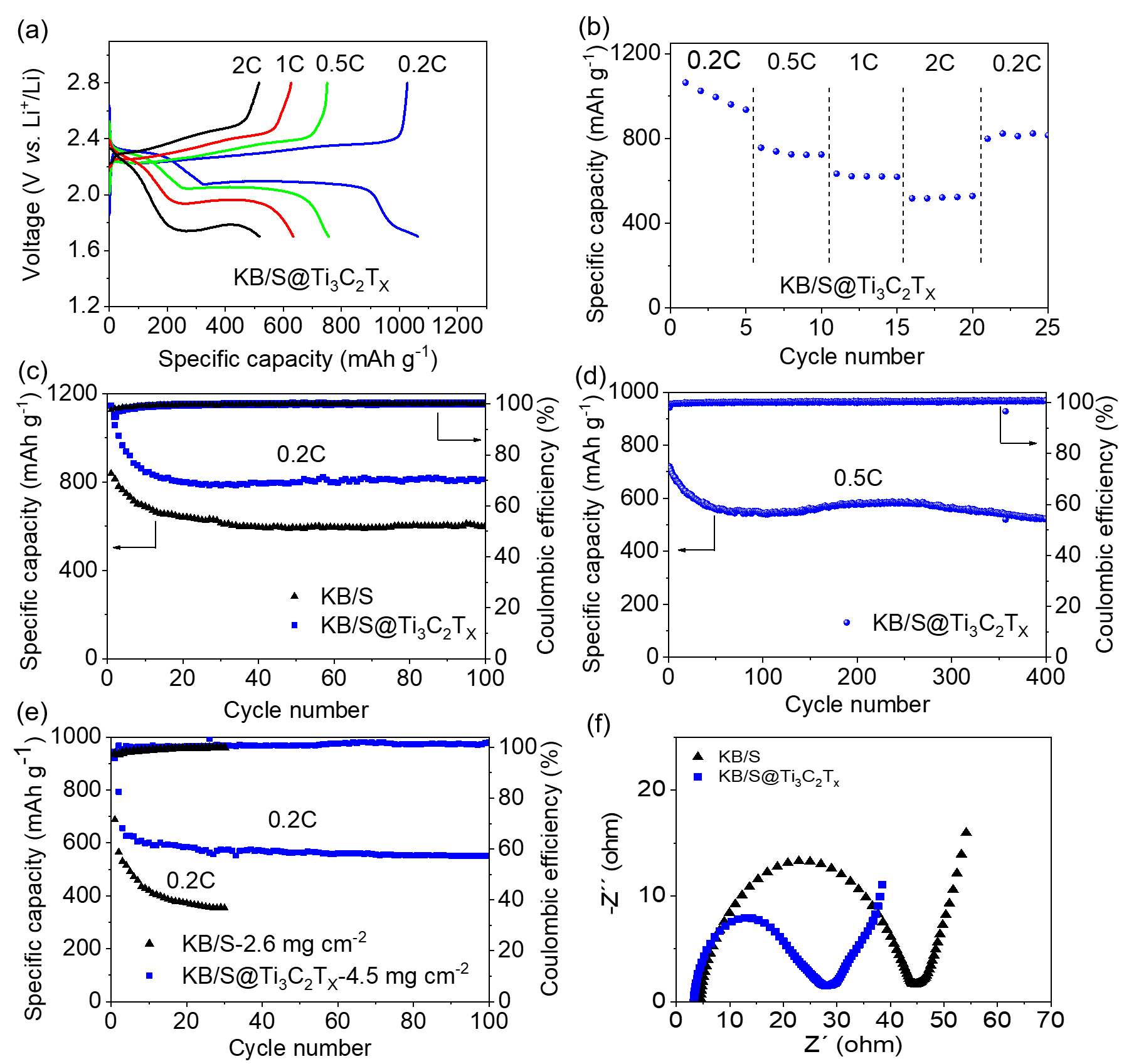
Figure 3. (a) The voltage curve of KB/S@Ti3C2Tx electrode at various magnifications in the range of 0.2 C to 2 C. (b) The rate performance of KB/S@Ti3C2Tx electrode. (c) Cycle performance of KB/S and KB/S@Ti3C2Tx electrodes at 0.2 C. (d) Long cycle of KB/S@Ti3C2Tx electrode at 0.5 C. (e) The cycling performance of KB/S and KB/S@Ti3C2Tx electrodes at a sulfur load of 2.6 mg cm-2 and 4.5 mg cm-2, respectively, at a rate of 0.2C. (f) Nyquist plots of KB/S and KB/S@Ti3C2Tx electrodes.
III KB@Ti3C2Tx coating further improves sulfur utilization
Inserting an intermediate layer between the separator and the positive electrode is an effective method to further hinder the shuttle of polysulfides. Figure 4a SEM picture shows that KB@Ti3C2Tx composite material is composed of MXene matrix and uniformly distributed KB. The increase of the surface area of KB@Ti3C2Tx composite material with interwoven structure ensures that it fully exposes the active sites. The composite material is applied to the diaphragm with a spatula. The SEM image in Figure 4b shows that KB/S@Ti3C2Tx particles are uniformly coated on the surface of the diaphragm. The surface load of KB@Ti3C2Tx is about 0.28 mg cm-2, and the thickness is about 3 μm, accounting for only 3% of the thickness of the sulfur electrode (Figure 4c). The KB@Ti3C2Tx intermediate layer is much thinner than most reported intermediate layers, too thin to significantly affect the volume/weight energy density of Li-S batteries. The prepared KB@Ti3C2Tx coating modified diaphragm can maintain excellent flexibility and mechanical strength, and there is no cracking and falling off when the folded shape is shown in Figure 4d. In order to verify that the KB@Ti3C2Tx coating modified separator has the ability to inhibit the shuttle of polysulfides, a visible H-type glass cell was assembled. Figure 3e shows the rapid diffusion of polysulfides through the unmodified membrane. The solution in the right container changed from colorless to pale yellow within 4 hours, indicating that the diffusion of polysulfides was not controlled. In sharp contrast, the H-type battery with KB@Ti3C2Tx coating modified separator only started to turn pale yellow after 8 hours in the right container.
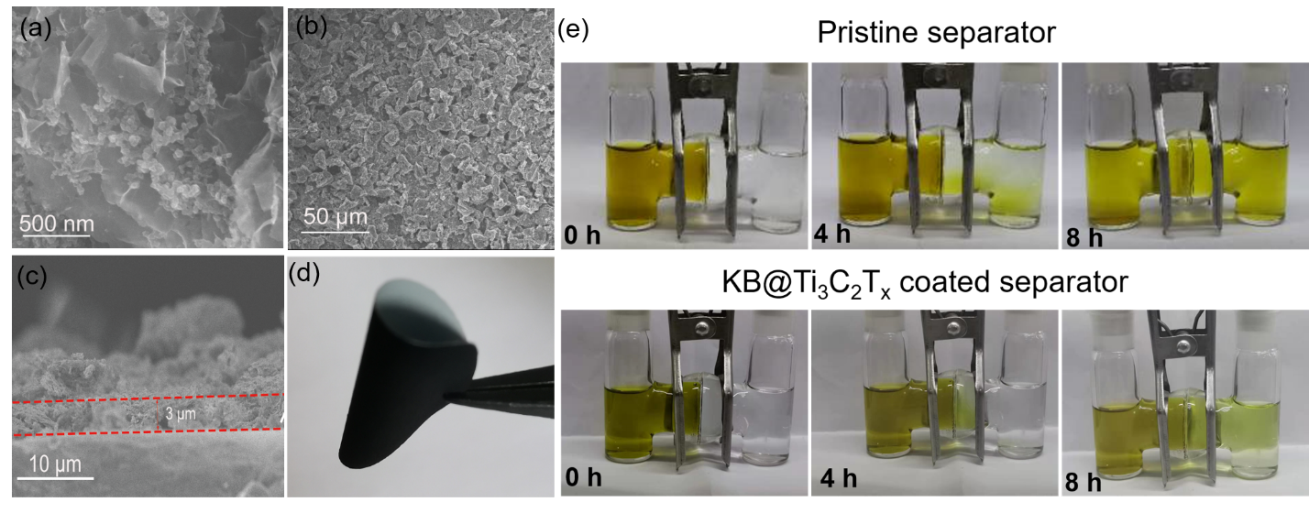
Figure 4. (a) SEM image of KB/S@Ti3C2Tx. (b) SEM image of KB/S@Ti3C2Tx coating modified diaphragm. (c) SEM cross-section of KB/S@Ti3C2Tx coating modified diaphragm. (d) Photograph of KB/S@Ti3C2Tx coating modified diaphragm. (e) Use unmodified PP diaphragm and KB/S@Ti3C2Tx coating modified diaphragm to measure polysulfide penetration.
Figure 5 shows the effect of KB@Ti3C2Tx modified separator on the electrochemical performance of Li-S battery. With and without modified separators, comparisons were made between batteries based on KB/S@Ti3C2Tx electrodes. Figure 5a Cyclic voltammetry (CV) measurement shows that KB@Ti3C2Tx coated diaphragm can increase the peak current of the cathode. The MXene in the intermediate layer inhibits the escape of polysulfides during the charging/discharging process, and provides additional reaction sites for the active material, thereby obtaining additional electron flow. The voltage curve in Figure 5b proves that the KB@Ti3C2Tx modified separator can significantly increase the specific capacity of the battery while also realizing better rate performance (Figure 5c). Figure 5d shows that the battery with KB@Ti3C2Tx modified separator also has better cycle performance at 1 C. The initial discharge capacity of the battery is 880 mAh/g, and after 400 cycles it still maintains a considerable capacity of 629 mAh/g, which is equivalent to an attenuation of 0.071% per cycle. Correspondingly, the area capacity and specific capacity at high sulfur loading are plotted in Figure 5e. Its discharge capacity is 1137 mA h/g at 0.05 C and 810 mAh/g at 0.2 C. The corresponding areal capacities are 6.4 mAh cm-2 and 4.5 mAh cm-2, respectively. After 100 cycles, the battery can still be Provides a capacity of about 600 mAh/g.
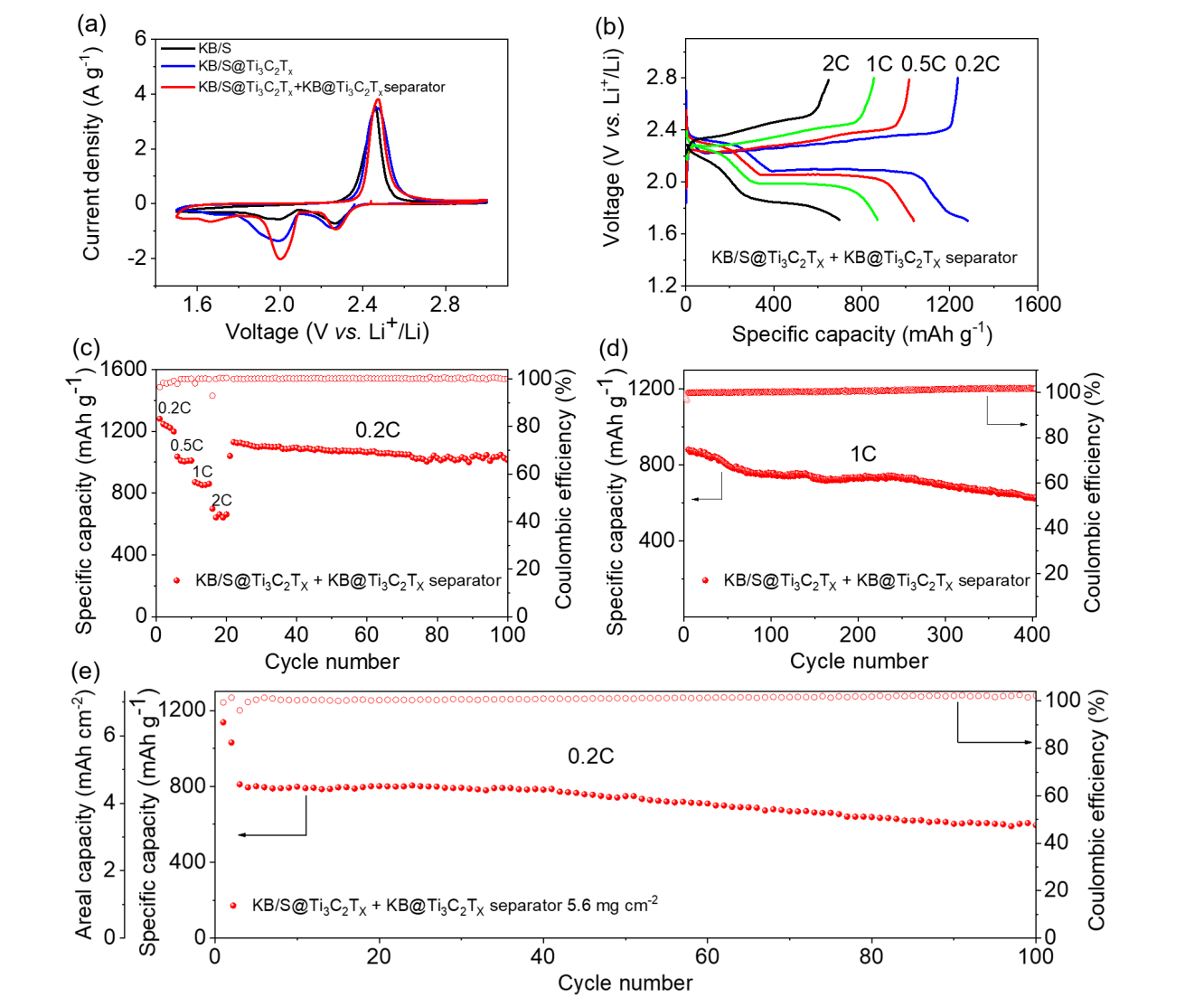
Figure 5. (a) The CV curve of an unmodified diaphragm and KB/S and KB/S@Ti3C2Tx electrodes, and the CV curve of a KB/S@Ti3C2Tx electrode with KB@Ti3C2Tx modified diaphragm. (B) Voltage curve of KB/S@Ti3C2Tx battery with KB@Ti3C2Tx modified separator, with a rate of 0.2 C to 2C, and (c) rate performance. (d) Long cycle performance of KB/S@Ti3C2Tx electrode with KB@Ti3C2Tx modified diaphragm at 1 C and (e) cycle performance under high sulfur load at 0.2 C and 5.6 mg cm-2.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately










