已传文件:photo/1631586161.png
Efficient and safe cell engineering by transfection of nucleic acids is still one of the long-term obstacles to basic biomedical research and many new therapeutic applications such as cart cell-based therapy. In recent years, mRNA has received increasing attention as a safer and more versatile alternative tool than virus or DNA transposon-based methods. However, the limitations of existing non-viral mRNA delivery pathways hinder the progress of genetic engineering that is difficult to transfect immune cells.
Intracellular Delivery of mRNA in Adherent and Suspension Cells by Vapor Nanobubble Photoporation
Laurens Raes, Stephan Stremersch, Juan C. Fraire, Toon Brans, Glenn Goetgeluk, Stijn De Munter, Lien Van Hoecke, Rein Verbeke, Jelter Van Hoeck, Ranhua Xiong, Xavier Saelens, Bart Vandekerckhove, Stefaan De Smedt, Kevin Koen Raemdonsck
Nano‑Micro Lett.2020 12:185
Highlights of this article
1. Vapor nanobubbles VNB light perforationIt is a promising physical technology for transfecting mRNA of adherent cells and suspension cells.
2. The parameters related to the VNB photoperforation process are optimized to achieve efficient mRNA transfection.
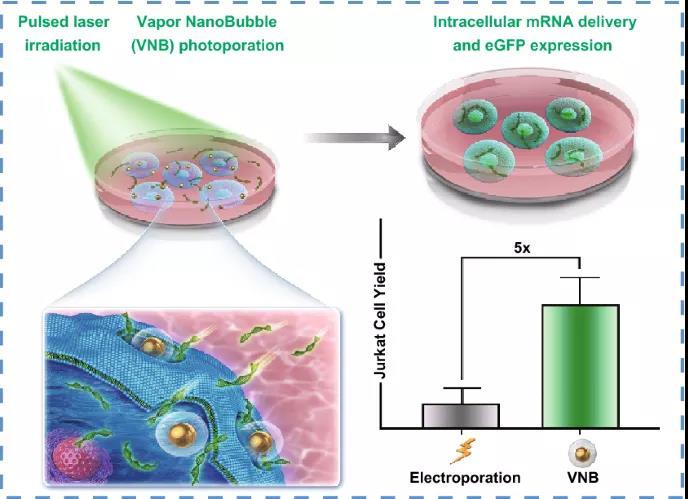
3. The in vivo transfection of Jurkat T cells produced by VNB photoporation is five times that of electroporation, that is, the current standard non-viral transfection technology for T cells is compared with electroporation.
In this study, Professor Kevin Braeckmans from Ghent University in Belgium and others demonstrated that gold nanoparticle-mediated vapor nanobubbles VNB photoperforation is a promising physical transfection method that can deliver mRNA in adherent cells and suspended cells . Preliminary transfection experiments on HeLa cells showed the importance of transfection buffer and carrier concentration, and this technology further proved that mRNA can be effectively delivered in Jurkat T cells with a transfection efficiency of up to 45%. Importantly, compared with electroporation, electroporation is a reference technique for non-viral transfection of T cells, and the number of live Jurkat T cells transfected has increased fivefold. All in all, the research results show that the VNB photoperforation technology as a more gentle and effective technology for intracellular mRNA delivery of adherent cells and suspension cells has the potential to transform cells in the future in therapeutic and basic research applications.
Graphic guide
VNB photoporous method for transfection of mRNA
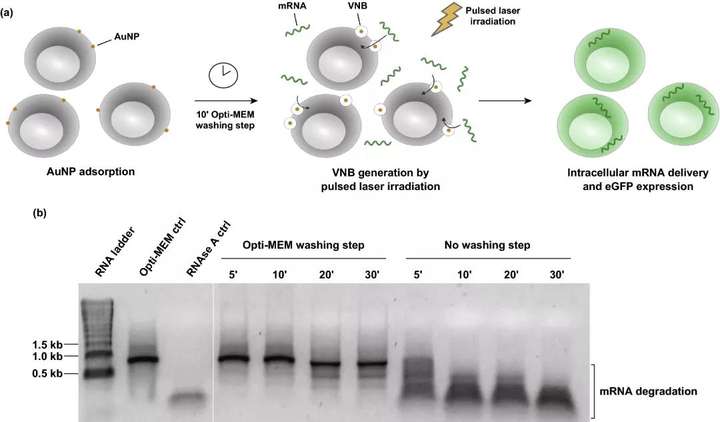
The applicability of AuNPs coated with 60 nm cationic PDDAC as photothermal nanoparticles for VNB photoperforation was studied. AuNPs with a diameter of 60 nm are considered to be ideal photothermosensitizers and require the smallest bubble nucleation threshold laser fluence. For VNB photoporous transfection, first incubate the cells with cationic AuNPs. After washing out the unbound AuNPs, irradiation with a single laser pulse 7 ns will cause the cell-bound AuNPs to produce VNBs. When heat energy is consumed, VNBs inevitably collapse, leading to the formation of local pores in the cell membrane, allowing extracellular mRNA molecules to diffuse directly into the cytoplasm through these membrane pores.
Figure 1. The optimized method of vapor phase nanobubbles VNB photoporous method for mRNA transfection. (a) Schematic diagram of mRNA transfection with VNB photopore method. (b) Analyze the integrity of physical mRNA by natural agarose gel electrophoresis; 1 μg eGFP mRNA was incubated on HeLa cells for a specific time, and washed with or without Opti-MEM in advance. As a control, mRNA was cultured only in Opti-MEM Opti-MEM-ctrl or incubated with 10 μg mL RNAse A RNAse A ctrl for 30 minutes.
II VNB photoporous method for transfection of mRNA in adherent cells
The HeLa cell line is used here as a reference cell type for initial optimization, because it has been widely used to quantify the delivery of various molecules siRNA and Nanobodies in cells through VNB photoporation. Optimized the relevant parameters of the VNB photoporous method, including AuNP concentration, laser energy, transfection buffer and mRNA concentration to achieve maximum transfection efficiency and acceptable cytotoxicity. Consistent with most scientific studies on adherent cell lines, the HeLa cell experiment chose a cytotoxicity threshold of 80%.
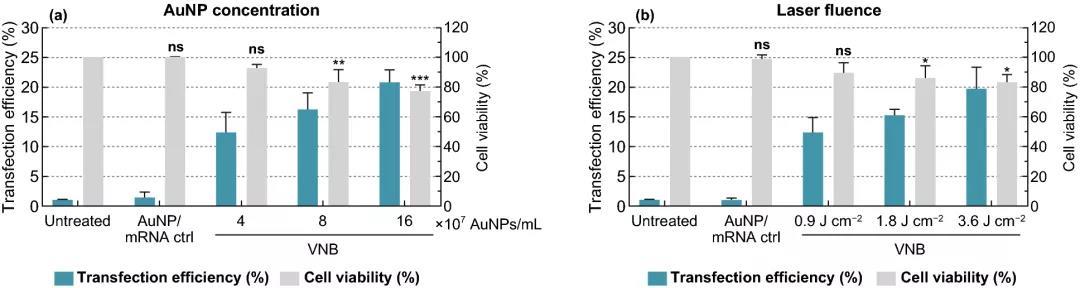
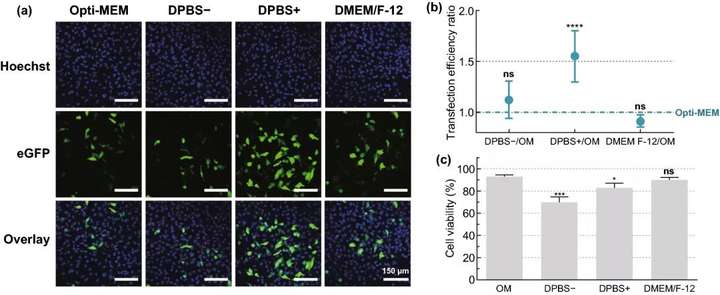
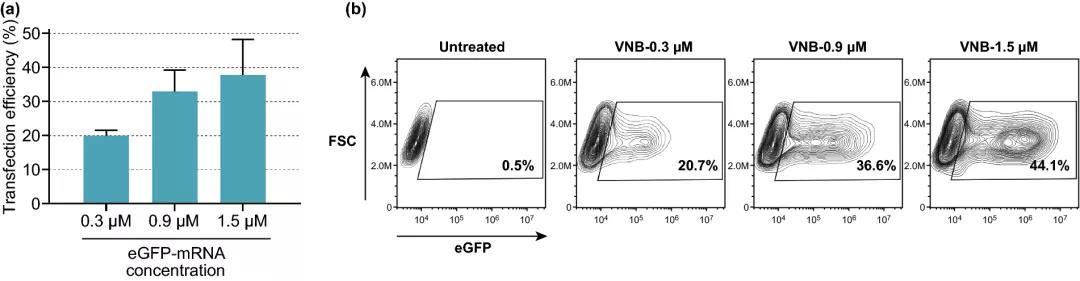
The experimental results show that when the cell viability is maintained above 80%, 8×10 AuNPs mL~5 AuNPs/cell is selected as the optimal concentration, which can produce about 16% of eGFP-positive cells; at 3.6 J cmUp to 20% of eGFP-positive cells can be produced under laser irradiation; when DPBS+ is used as the transfection buffer for HeLa cells, the transfection effect is best; with the increase of mRNA concentration, the percentage of eGFP-positive cells increases, 1.5 μM mRNA The proportion of eGFP-positive cells reached 38%.
Figure 2. Gold nanoparticle AuNP concentration and laser irradiation optimized eGFP-mRNA transfection of HeLa cells. (a) In the presence of 0.3 μM-eGFP-mRNA, a fixed laser energy of 1.8 J cm and different concentrations of AuNP were used to photopororize HeLa cells. (b) In the presence of 0.3 μM eGFP-mRNA, a fixed AuNP concentration of 8×107 AuNP mL and laser energy of different intensities were used to photopororize HeLa cells. The transfection efficiency was expressed by the percentage of eGFP positive cells determined by flow cytometry n≥3. The cell viability value was measured by CellTiter-Glo and expressed relative to the untreated control group n=3. One-way analysis of variance and Dunnetts multiple comparison test were used to determine statistical differences ns: no significant difference; p<.05; p<.01; p<.001.
Figure 3. Screening of HeLa cell mRNA transfection buffer. Use different transfection buffers: Opti-MEM, DPBS-, DPBS+ or DMEM/F-12, by VNB photoporous method 0.3 μM mRNA; 8×107 AuNPs mL; 3.6 J cm Transfect HeLa cells. (a) A typical confocal microscope image of HeLa cells 24 hours after transfection scale bar=150 μm. (b) Flow cytometry n=6 was used to determine the transfection efficiency ratio of different buffers and OM. A one-way analysis of variance and Dunnetts multiple comparison test were performed to determine the statistical difference between OM and other buffers ns=not significant; p<.0001. (c) Cell viability at 24 hours after transfection, compared with the untreated control group n=3. A one-way analysis of variance and Dunnetts multiple comparison test were performed to determine the statistical difference between OM and other buffers ns=not significant; p<.05; p<.001.
Figure 4. The effect of increasing eGFP-mRNA concentration on transfection efficiency. (a) The transfection efficiency of eGFP-mRNA at different concentrations, expressed as the percentage of eGFP-positive cells (n=3). (b) Contour map of a typical experiment on eGFP-mRNA transfection of HeLa cells.
III eGFP‑mRNA transfection of Jurkat T cells by VNB photopore method
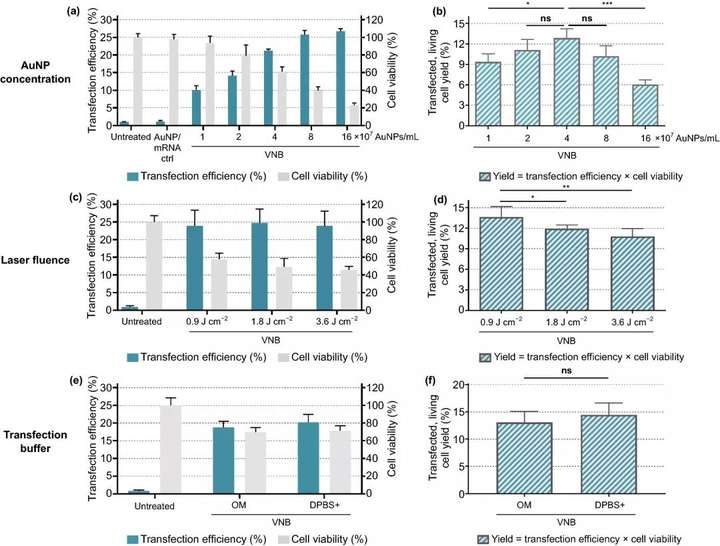
The Jurkat E6-1 human leukemia T cell line was used as the primary human T cell model. Similar to the HeLa cell transfection experiment, the different key parameters in the VNB photoporation process of Jurkat cells were optimized Figure 5, namely: (1) AuNP concentration; (2) laser fluence; (3) transfection Buffer. The experimental results show that the concentration of 4×10AuNPs mL ~2 AuNPs/cell is the best condition. Under this condition, about 13% of the initial cell population is viable and transfected; at 0.9 J cm Under the lowest laser energy, the yield of live cells and transfected cells is the highest ~14%; using OptiMEM as the transfection buffer provides the best transfection efficiency on Jurkat cells.
Figure 5. Optimization of gold nanoparticle AuNP concentration, laser energy and transfection buffer for eGFP-mRNA transfection into Jurkat cells. Transfection efficiency represents the percentage of eGFP-positive cells, cell viability value is calculated relative to the untreated control group, and yield is the product of transfection efficiency and cell viability. a, b Using a fixed laser fluence of 1.8 J cm to screen AuNP concentration n=3, using Dunnetts multiple comparison test for one-way analysis of variance, ns=not significant; p<.05,p<.001. c, d Use a fixed AuNP concentration of 4×10AuNP mL to screen laser energy unit: J cm n=3, using one-way analysis of variance of Dunnett multiple comparison test, ns=meaningless; p <.05, p<.01). e, f Compare the transfection efficiency of Opti-MEMOM and DPBS+ as transfection buffer, using a fixed AuNP concentration of 4×10 AuNPs mL and a laser energy of 0.9 J cm n=3, unpaired Studens T test, ns=not significant)
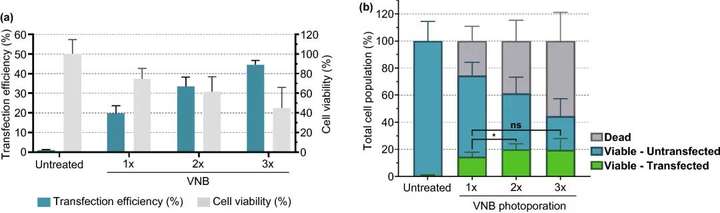
Figure 6. The effect of multiple consecutive VNB light hole treatments on the mRNA transfection efficiency of Jurkat cells. (a) The transfection efficiency represents the percentage of eGFP-positive cells, and the cell viability value is calculated relative to the untreated control group. (b) Calculate the number of live cells/transfected cells, live cells/untransfected cells and dead cells under different conditions n≥3, single-factor analysis of variance and Tukeys multiple comparison test, p<0.05, ns=none Significance.
IV VNB photoperforation produces more viable mRNA than karyotype infection to transfect Jurkat cells
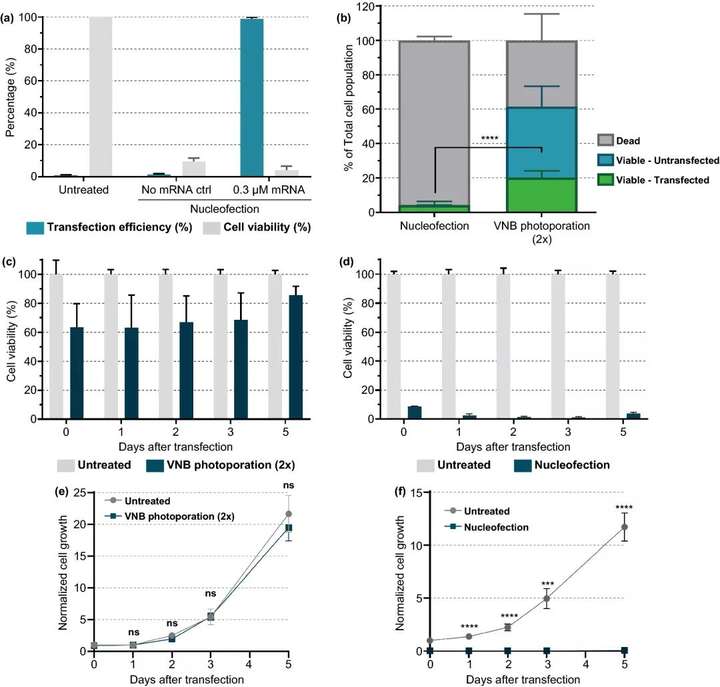
Electroporation is currently the most commonly used non-viral nucleic acid transfection and T cell in vitro modification technology. Comparing our technology with commercial electroporation systems, the results show that the number of live Jurkat T cells transfected has increased fivefold.
Figure 7. Comparison of karyotype infection and VNB photoporous mRNA transfection of Jurkat cells. (a) Transfect eGFPmRNA 0.3 μM into Jurkat cells by nuclear infection according to the manufacturers instructions. 24 h =3after transfection, the transfection efficiency that is, the percentage of eGFP positive cells and cell viability were determined by the CellTiter-Glo method. (b) Comparison of the yield of live cells and transfected cells between karyotype infection and VNB photopore method n≥3, unpaired Students T test; p<0.0001. c, d 5 days after transfection, measure the cell viability of VNB light hole (c) or nuclear infection (d) at different time points. On day 0, cell viability was measured 2h after transfection. The cell viability value compared with the untreated control group was calculated every day . (e, f) Five days after transfection, the normal cell growth after VNB light hole (e) or nuclear infection (f) was measured at different time points. On day 0, the normalized cell growth value compared with the untreated control group was calculated. Data are expressed as mean±SEM n=2×3, unpaired Students t test T; ns=not significant, p<0.001, p<0.0001.
About the Author
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately