已传文件:photo/1631586161.png
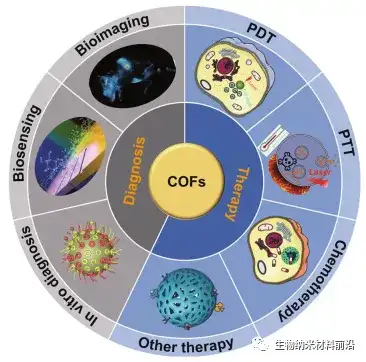
1. Article overview
Covalent organic framework (COF), as a porous crystalline covalent organic polymer, is composed of covalently connected and periodically arranged organic molecules. Their precise assembly, well-defined coordination network and adjustable porosity give COF a variety of characteristics, such as low density, high crystallinity, porous structure, large specific surface area, and multifunctional functions and activities that can be adjusted on the molecule Sites and so on. These unique properties make them excellent candidate materials for biomedical applications, which can be used in the fields of drug delivery, diagnostic imaging, and disease treatment. The composition, size, and load of guest molecules in COF make them useful in various applications. Performance has a big impact. In this review, we first introduced the effects of size, building blocks, and synthesis conditions on chemical stability, pore structure, and chemical interaction with COF guest molecules; then, we summarized the application of COFs in cancer diagnosis and treatment; Finally, it points out some challenges of COFs in cancer treatment, and puts forward the problems that need to be solved in the future.
Two, graphic guide
Figure 1. COFs material classification.
Figure 2. COF materials with different chemical stability. (A) The construction of binary, ternary and quaternary COFs and their stacking modes. (B) Schematic diagram of rigid and curved HBC nodes and their combination in borate COF to obtain Marta-COF-1 with a wavy and complementary structure that guides the stacking of COF layers. (C) Common skeletons without alkyl groups will collapse when exposed to harsh environments, while hydrophobic skeletons with alkyl groups can withstand harsh environments due to the protection of hydrophobic groups.
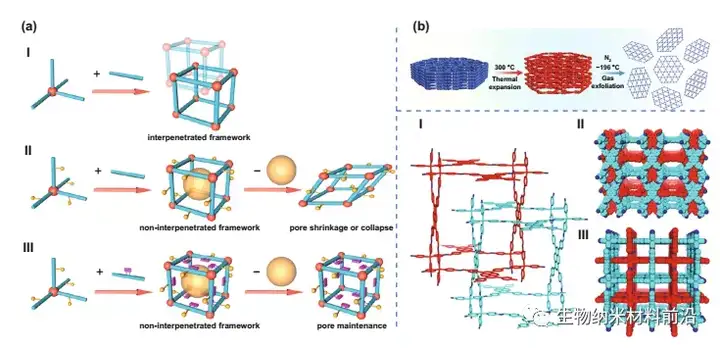
Figure 3. (a) COFs with different pore structures and their chemical interactions with guest molecules. Ⅰ Interpenetrating microporous frame constructed by unmodified edges; Ⅱ Non-interpenetrating mesoporous frame constructed by partially decorated edges, in which pore shrinkage or collapse occurs after removal of guest molecules; Ⅲ Created by highly decorated edges Non-interpenetrating mesoporous framework, in which the pores can be completely maintained after removing the guest molecules. (B) Schematic diagram of variable temperature gas exfoliation of NUS-30 from massive layered powder to ultra-thin two-dimensional nanosheets. (C) I dual-interconnected PTS network; II see the space filling model from the A axis; III see the space filling model from the C axis.
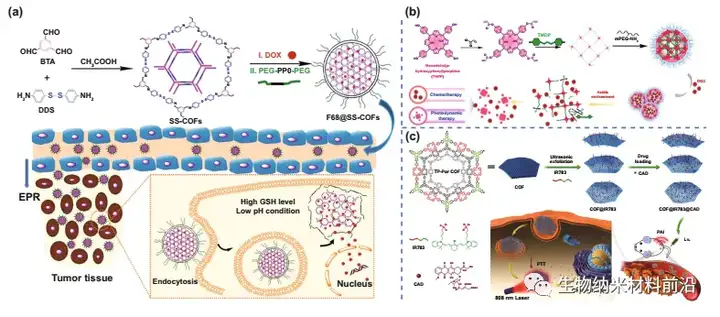
Figure 4. COF for drug delivery. (A) Schematic diagram of the preparation of drug-loaded F68@SS-COFs and its intracellular GSH-responsive drug release. (B) The preparation of drug-loaded F68@SS-COFs and the mechanism of GSH-responsive drug release in cells. (C) Schematic diagram of nanocomposite material preparation and cancer treatment process in vivo.
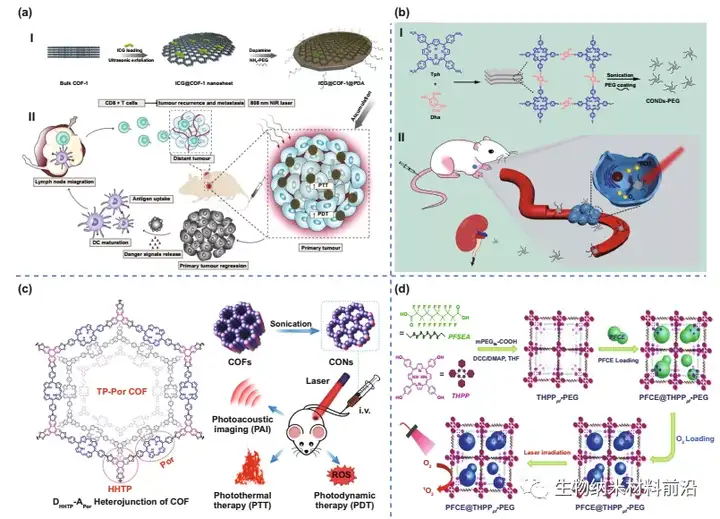
Figure 5. COF materials used to mount PDT. (A) Preparation process (Ⅰ) and photoimmunotherapy activity of ICG@COF-1@PDA nanosheets (Ⅱ). (B) The preparation process of COF-PEG nanodots. (C) Schematic diagram of 2D CON preparation and in vivo cancer treatment. (D) Illustrate the synthetic route and PFCE loading of THPPpf-PEG.
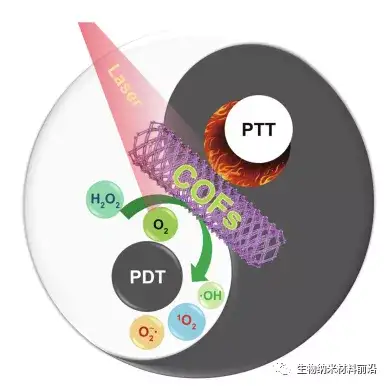
Figure 6 Schematic diagram of COF light therapy.
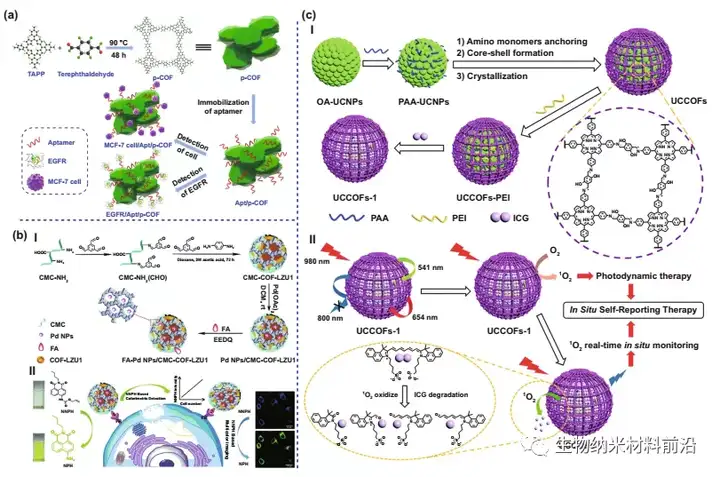
Figure 7. COF for imaging and diagnosis. (A) Schematic diagram of p-COF-based aptamer sensor for detecting EGFR or MCF-7 cells, including 1) preparation of p-COF; 2) fixation of aptamer chain; and 3) EGFR or MCF-7 Of detection cells. (B) Schematic diagram of the synthesis process of ⅠFA-Pd NPs/CMC-COF-LZU1; Ⅱ Schematic diagram of the dual-function FA-Pd NPs/CMC-COF-LZU1 for cancer cell imaging. (C) Schematic diagram of the design and synthesis of the ICOF nanoplatform UCCOFs-1; schematic diagram of the in-situ self-reporting PDT process of ⅡNIR excitation.
3. Full text summary
In this review, we outline the recent breakthroughs in the application of COF materials to cancer diagnosis and treatment. In view of their versatility and physical and chemical properties, COFs show unique advantages in cancer diagnosis and treatment, especially in drug delivery, phototherapy (including PDT and PTT), biosensing and bioimaging. With its high surface area and porous properties, COF also shows unique advantages in delivering anti-tumor drugs to the desired location. For PDT and PTT, COFs can be used not only as the carrier of PSs or PTAs, but also as the photosensitive group of PSs or PTAs due to their high light absorption cross section, π-conjugated and multifunctional structure. COFs can effectively avoid the annihilation of PSs caused by π-π stacking, and has good thermal stability, high contact area and light utilization. These characteristics also help COF play a role in bioimaging and diagnosis, as well as imaging-guided therapy. All in all, compared with other porous nanomaterials, nano-level COFs show unique advantages in the field of cancer diagnosis and treatment. Unlike most other porous nanomaterials that only serve as inactive carriers for drug delivery and achieve other functions only through functionalization after synthesis, the inherent electrical, magnetic and optical properties of COF itself can be directly used for therapeutic purposes, drug delivery and other treatment methods (Including phototherapy and immunotherapy) synergy, which can solve many challenges of cancer treatment, including early diagnosis difficulties, drug resistance, recurrence and metastasis.
Article link:
https://doi.org/10.1007/s40820-021-00696-2
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.