已传文件:photo/1631586161.png
Beiconn can provide cobalt-doped NiMn-LDH)/V₂CTₓ MXene (customizable)
Research abstract

High-performance aqueous electrochemical energy storage technology has attracted extensive attention due to its high safety and commercialization potential. Recently, the research team of researcher Wang Lili from the Beijing Institute of Semiconductors, Chinese Academy of Sciences published a research paper entitled "Self-assembled Cobalt-doped NiMn-Layered double hydroxide (LDH)/V2CTx MXene hybrids for advanced electrochemical energy storage properties" in the internationally renowned journal Chemical Engineering Journal. Research Papers. In this paper, 2D/2D cobalt-doped NiMn layered double hydroxide (LDH)/V2CTx MXene (CNMV) electrode materials were successfully synthesized by a simultaneous doping-electrostatic synergistic assembly strategy for use in aqueous supercapacitors and zinc-ion batteries. As expected, the CNMV electrode provided a high specific capacitance of 1005 F g-1 at 1 A g-1 and 30.16 Wh kg-1 at 700 W kg-1 after assembly into an asymmetric supercapacitor (ASC). High energy density. Furthermore, for aqueous Zn-ion batteries, the CNMV cathode exhibits a high capacity of 322.7 mAh g-1 after 100 cycles at a current density of 0.2 A g-1, as well as an excellent rate capability (65.6 mA at 5 A g-1). h g-1). The energy storage mechanism of CNMV electrodes for supercapacitors and zinc-ion batteries was evaluated by quantitative kinetic analysis. This study provides a facile and efficient method for designing high-performance MXene electrodes for aqueous electrochemical energy storage devices.
Graphical guide
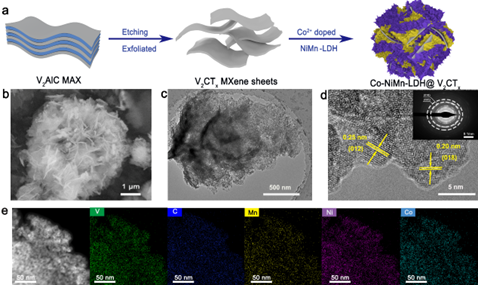
Figure 1. Preparation process and morphology analysis of CNMV composites.
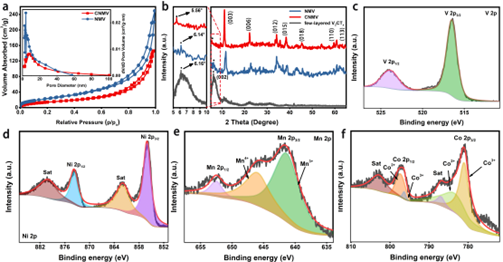
Figure 2. Structural characterization of CNMV complexes.
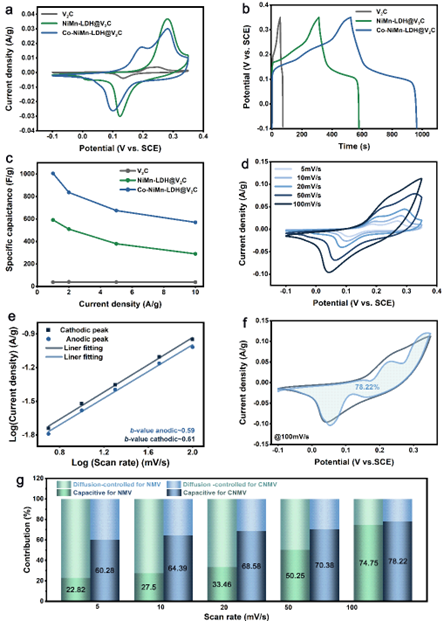
Figure 3. Electrochemical performance of CNMV, NMV and few-layer V2CTx for supercapacitors.
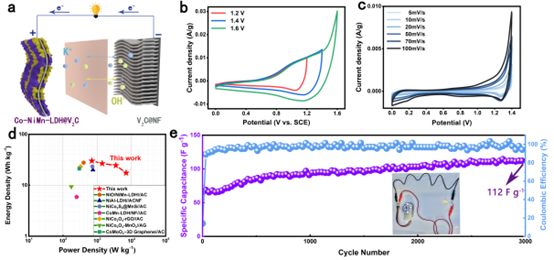
Figure 4. Performance comparison. Preparation and electrochemical performance of CNMV//V2C ASC devices.
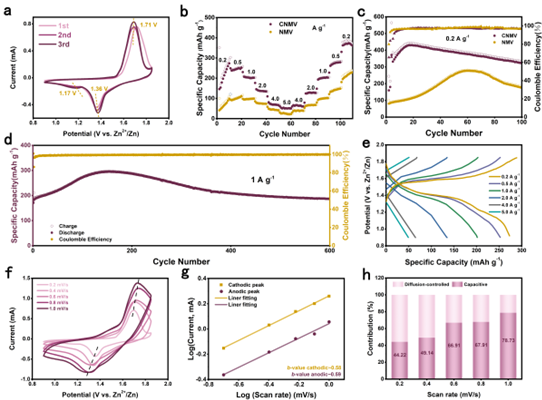
Figure 5. Electrochemical performance of NMV and CNMV for aqueous ZIB.
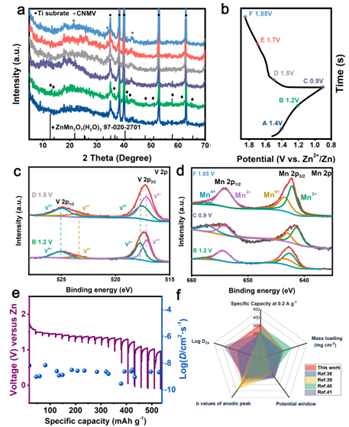
Figure 6. Analysis of zinc ion storage mechanism of CNMV cathode.
Summarize
Point 1: The CNMV electrode material was successfully synthesized by the simultaneous doping-electrostatic synergistic assembly strategy.
Two-dimensional (2D) transition metal carbides/nitrides (MXenes) have attracted extensive attention due to their unique physicochemical properties, such as metallic conductivity and good electrochemical charge storage capacity. Meanwhile, two-dimensional layered double hydroxides (LDHs) have also been considered as promising electrode materials for energy storage devices due to their high theoretical capacitance. However, low conductivity and aggregation during electrochemical ion storage lead to a dramatic capacity drop, as well as poor stability and rate performance during long cycling, hindering its commercial application. Here we fabricated CNMV electrodes by a simultaneous doping-electrostatic assembly strategy, with negatively charged 2D V2CTx MXene as substrate providing high conductivity, while positively charged Co-doped NiMn-LDH provided abundant electrochemical activity and suppressed the cycling process reunion in.
Point 2: It has excellent electrochemical properties for supercapacitors and can be assembled into high energy density ASC devices
Due to the effect of hetero-ion doping, the CNMV electrode has the largest integrated area of the CV curve and has two pairs of highly reversible redox peaks, which are related to the M(OH)2/MOOH (where M = Co, Ni or Mn) Reversible Faradaic reaction behavior. CNMV electrodes have a high specific capacitance of 1005 F g-1 at 1 A g-1. From the trend of contribution rate, NMV electrodes are dominated by diffusion-controlled charge storage. While for CNMV, the capacitive contribution dominates, and the battery-type electrode transforms into a pseudo-capacitive electrode, indicating that the effect of hetero-ion doping changes the energy storage mechanism. In addition, an asymmetric supercapacitor ASC device assembled with CNMV sample as cathode and multilayer V2CTx MXene as anode achieves a high energy density of 30.16 Wh kg-1 at a power density of 700 W kg-1.
Point 3: It has excellent properties when applied to water-based ZIB cathode materials.
The CNMV cathode exhibits a higher discharge capacity of 322.7 mA h g-1 after 100 cycles at a current density of 0.2 A g-1, and the long-cycle performance was tested for 600 cycles at a current density of 1 A g-1 , the discharge capacity is stable at 170 mAh g-1, and the capacity retention rate is 95.7%. Notably, a trend of capacity increase was observed in the first 100 cycles, stemming from the lack of a pre-cycle activation process during long-term cycling. However, CNMV has a shorter activation process due to the improved structural stability due to the hetero-ion doping effect. The electrochemical storage process of CNMV cathodes is governed by capacitive and diffusive reactions, in which the diffusion-controlled behavior dominates, indicating the fast charge transfer kinetics of the Zn ion storage mechanism at higher scan rates and the rapid charge transfer kinetics due to the hetero-ion doping effect. Excellent rate performance.
Point four: Zn ion energy storage mechanism analysis.
To investigate the electrochemical process and structural evolution of CNMV cathodes during Zn intercalation/intercalation, we performed ex situ XRD and XPS analyses. The ZnMn3O7(H2O)3 phase appeared at the discharge voltage of 1.2 V in the first cycle and gradually disappeared during the next charging process due to the intercalation of Zn2+. When charged to 1.5 V in the next cycle, the peaks return to their original positions, indicating that the structural evolution during charge-discharge is completely reversible with good structural stability. The valence state of Mn is mainly composed of Mn3+ and Mn4+, indicating that the oxidation reaction is not complete, which is related to the effect of hetero-ion doping. From 1.2 V discharge to 0.9 V full discharge, the Mn 2p3/2 peak shifts significantly to lower binding energies due to the reduction of Mn4+ to Mn3+ by the intercalation of Zn2+ and H+. However, Mn 2p2/3 recovered to the original peak at the next charging cycle, indicating a high oxidation of Mn. The change of manganese valence states illustrates the reversibility of the Zn2+ intercalation/intercalation process.
Literature link
DOI: 10.1016/j.cej.2021.132992
For the original text, please click the lower left corner of the tweet to read the original text
【Introduction to the corresponding author】
Wang Lili is a researcher at the Institute of Semiconductors, Chinese Academy of Sciences, and a high-level talent of the Chinese Academy of Sciences. In 2017 and 2018, she was selected into the "Young Talent Support Project" and "Future Female Scientists" program of China Association for Science and Technology, respectively. In 2014, he received his Ph.D. in Microelectronics and Solid State Electronics from Jilin University. He then did postdoctoral research at Nanyang Technological University, Singapore. After returning to China in 2017, he is mainly engaged in the research of flexible bionic electronic devices and integrated systems in the fields of biomedicine and environmental monitoring. In the past 5 years, as the first and corresponding author, he has published more than 50 SCI papers in Chem. Soc. Rev, Adv. Mater., Adv. Funct. Mater., ACS Nano, Nano Energy and other journals, and 9 papers have been selected as ESI Global High Award Cited/hot papers, more than 4000 times in total, H-index 40. He also serves as the Young Editor/Guest Editor of J. Semicond., J. Phys. D., InfoMat and other journals.
【Introduction to the first author】
Zhang Yuming, received a bachelors degree in physics from Jilin University in 2018, and is currently a doctoral student in the 2020 class of the School of Physics, Jilin University. The research direction is the research and design of electrochemical energy storage devices based on 2D MXene materials.











