已传文件:photo/1631586161.png
North Konami can provide MXene (can be customized)


In the past few years, research on next-generation battery technologies (beyond Li-ion batteries) has developed rapidly. The key challenge for these emerging batteries is the lack of suitable electrode materials, which severely limits their further development. MXenes, as a new class of 2D transition metal carbides/nitrides/carbonitrides, are considered as potential electrode materials for these emerging batteries with some desirable properties. Large and controllable interlayer spacing, excellent hydrophilicity, ultra-high conductivity, synthetic diversity, and rich surface chemistry make MXenes not only potential electrode materials, but also emerging batteries. in other parts. Recently, Professor Husam N. Alshareef of King Abdullah University of Science and Technology in Saudi Arabia published a review article entitled MXenes for Rechargeable Batteries Beyond the Lithium-Ion in Advanced Materials, a top international material journal, summarizing the application of MXenes in various rechargeable batteries Research progress in , including alkali metal ion batteries (Na+, K+), multivalent ion (Mg2+, Zn2+ and Al3+) batteries, etc. In particular, the synthesis strategies and the different roles of MXenes in batteries, such as electrodes, protective layers of metal anodes, sulfur hosts, separator improvements, and conductive additives, etc., are discussed, and the future development is prospected.
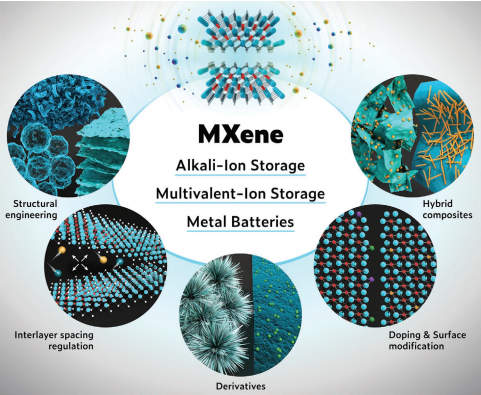

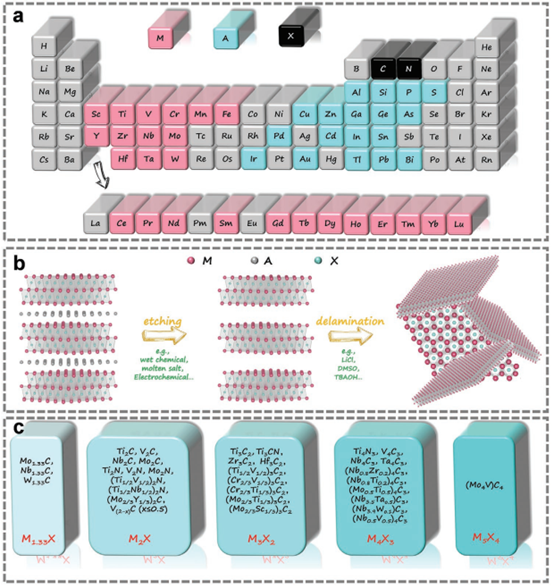
Figure 1. Composition of MAX and MXene.

Table 1. Two methods for synthesizing MXenes and experimental parameters.
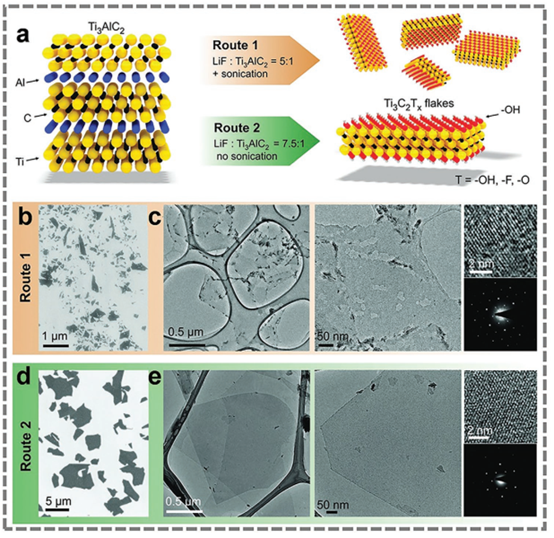
Figure 2. Different etching methods.
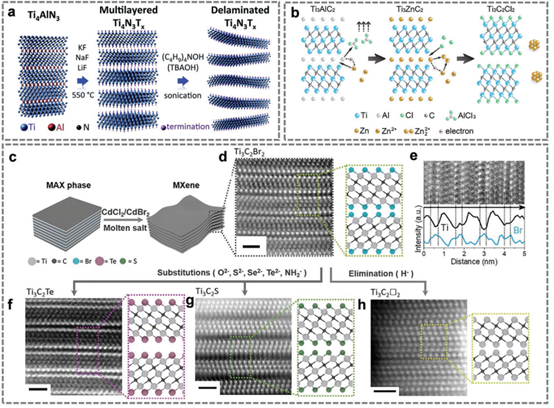
Figure 3. Molten salt etching of Ti4N3Tx; ZnCl2 molten salt etching of Ti2C2Cl2; Lewis acid molten salt etching of MAX phase.
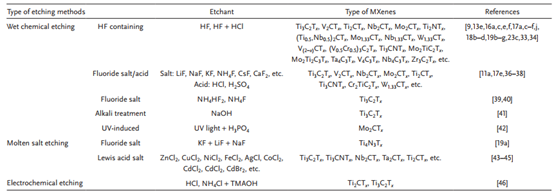
Table 2. Summary of different etching methods.
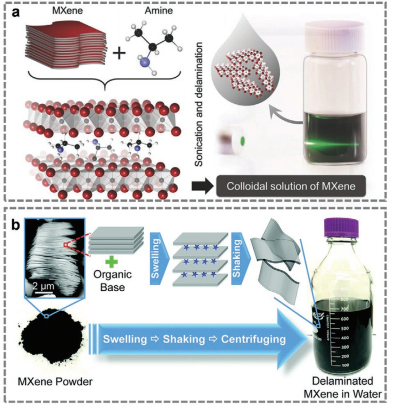
Figure 4. Synthesis of Nb2CTx.
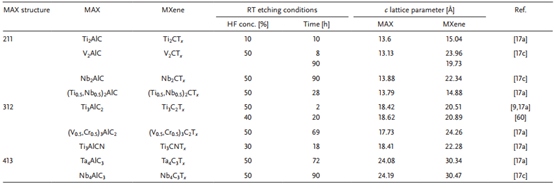
Table 3. Summary and comparison of different MAX phases and lattice parameters of MXenes.
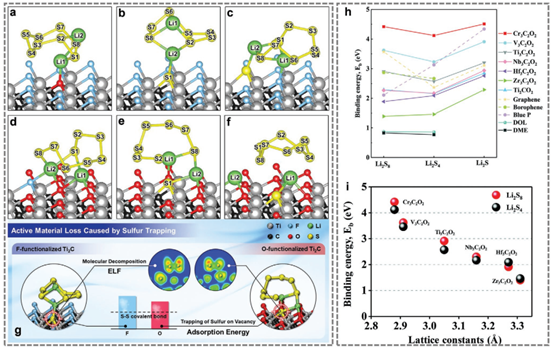
Figure 5. Theoretical calculations of MXenes in lithium-sulfur battery applications.
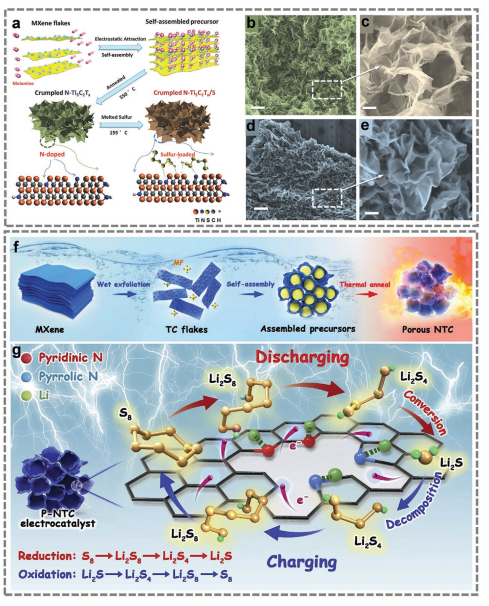
Figure 6. Preparation process of N-Ti3C2Tx@S electrode.
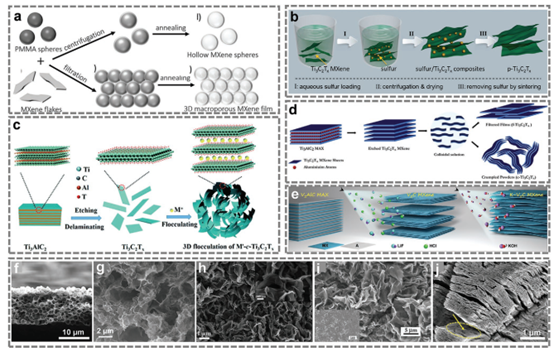
Figure 7. 3D, porous and wrinkled MXene preparation process.
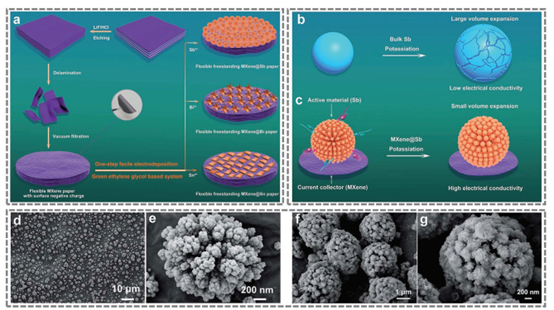
Figure 8. MXene@metal (Sb, Bi and Sn) flexible self-supporting electrodes.
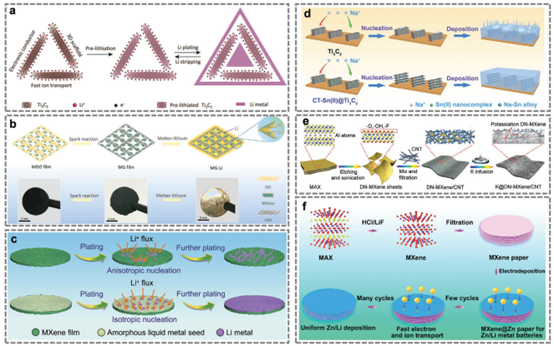
Figure 9. Preparation process of some typical MXene-based electrodes.

The high electrical conductivity and rich surface chemistry of MXenes have great potential to play different roles in batteries. Although the research on MXenes has made great progress in recent years, some challenges still exist, which need to be solved by researchers.
1) At present, the main synthesis methods of MXene all use HF, which is harmful and toxic to the experimenter. Exploring low-cost, efficient and environmentally friendly etching methods and using other etchants that are safer than HF is particularly important for large-scale high-quality MXene preparation. Among them, Lewis acid molten salt method and electrochemical method are very promising methods. In addition, the synthesis method also affects the conductivity of MXene materials, and exploring new etchants with less impact on the environment can further improve the conductivity of MXene.
On the other hand, bottom-up methods such as CVD can efficiently synthesize metal carbides directly and thus may also be a promising new method for the synthesis of high-quality MXenes.
2) The rich surface chemistry of MXenes has important effects on aspects such as electrochemical performance. These functional groups can selectively react with specific compounds, thus affecting the energy storage performance. The control of surface functional groups remains a challenge. Careful choice of etchant and etch method can help.
3) MXenes generally suffer from rapid oxidation problems in air and water. On the one hand, surface oxides enhance the electrochemical activity, but on the other hand, their electrical conductivity decreases and complicates the study of the intrinsic properties of MXenes.
4) MXene-based electrodes often exhibit pseudocapacitive properties. Due to their high electronic conductivity, MXene electrodes generally exhibit outstanding rate capability, making them ideal for hybrid ionic capacitors.
5) Although MXenes have been intensively studied in the field of energy storage, the ion dynamics and charge storage behaviors in different energy storage devices (especially K+/Zn2+/Al3+ batteries, etc.) are not comprehensive enough. This facilitates the design and optimization of MXene-based electrodes.
6) Although more than 30 MXenes have been synthesized in the laboratory, the most in-depth research is still the first MXene, Ti3C2Tx, although it exhibits unique and potential properties, it does not mean that it is suitable for The best choice in all applications.
Literature link:
DOI: 10.1002/adma.202004039



























