已传文件:photo/1631586161.png
North Konami can provide MXene (can be customized)


Research abstract
Two-dimensional transition metal carbides or nitrides (MXenes) with high electrical conductivity, hydrophilicity, large specific surface area, and tunable surface properties have shown great potential in electrocatalysis. At present, the most widely used preparation method for MXenes is to etch the A-layer atoms of the MAX phase (A is an element of the III and IV main groups) with HF or LiF+HCl. Although under the premise that the surface functional groups are O or OH groups, theoretical predictions confirm that some pure MXenes are promising HER catalysts. However, the experimental results show that the catalytic activity of pure MXenes is poor, which is generally due to the introduction of surface functional groups such as F and Cl during the preparation of MXenes by wet chemical etching of the precursor MAX phase. In addition, more active monolayer MXenes often require intercalating agents to achieve ultrasonic exfoliation. The extensive use of hydrofluoric acid and intercalating agents inevitably draws attention to laboratory safety and human health.
To overcome this bottleneck, researchers are also actively exploring various mild strategies for the preparation of fluorine-free MXenes, but the subsequent purification procedures inevitably bring trouble. However, despite the emerging applications of derived MXenes in the field of electrocatalysis, there are few reports on the electrochemical performance of MAX itself. Here, for the first time, the intrinsic HER performance of MAX has been systematically investigated and improved by a two-step in situ electrochemical synthesis method.
Introduction
The team of Professor Que Wenxiu of Xian Jiaotong University took the MXene precursor Mo2TiAlC2 MAX (a double transition metal-ceramic phase) as the research object, used in-situ electrochemical etching to pretreat the MAX electrode, and applied anodic current in 0.5 M H2SO4 electrolyte. Etching the Mo2TiAlC2 MAX phase, in just a few hours, yielded lower HER performances superior to most reported pure MXenes. This is due to the construction of a layer of amorphous carbide MAC on the surface of MAX, which improves the intrinsic catalytic activity of MAX. In addition, the formation mechanism and corrosion mechanism of MAX surface-derived surface MAC were revealed by ex situ XPS. Then, the highly active Pt nanoparticles were introduced to construct a heterocomposite interface with the surface of the carbide MAC after electrochemical etching, thereby achieving stable high-current electrocatalytic HER performance.
The result was published online in the top international journal Small (impact factor 15.153), titled: In Situ Electrosynthesis of MAX-Derived Electrocatalysts for Superior Hydrogen Evolution Reaction.
Sheng Minhao is the first author of this article.
Graphical guide
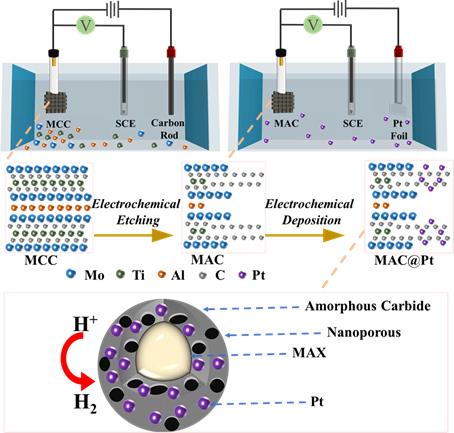
Figure 1. Schematic diagram of electrochemical synthesis of MAC@Pt.
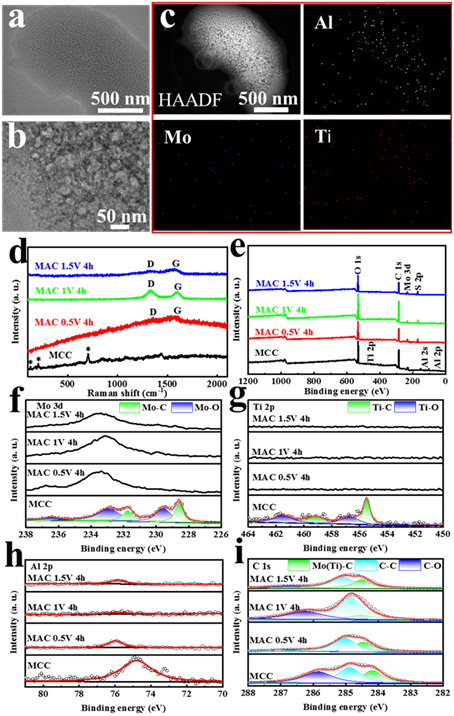
Figure 2. Structural characterization of the MAC. (a) TEM image of MAC 1 V 4 h. (b) HRTEM image of MAC 1 V 4 h. (c) HAADF-STEM image and element profile of MAC 1 V 4 h. (d) Raman spectra of MCC and 0.5-1.5 V 4 h MAC. (e) XPS of MCC and 0.5-1.5 V 4h MAC. (f) Mo 3d high-resolution XPS. (g) Ti 2p high-resolution XPS. (h) Al 2p high-resolution XPS. (i) C 1s high-resolution XPS.
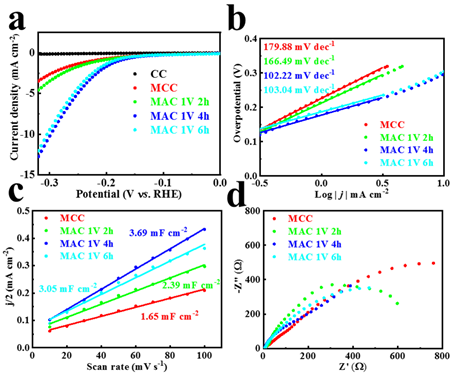
Figure 3. MAC electrochemical performance. (a) LSV curve. (b) Tafel slope. (c) Cdl values. (d) Electrochemical impedance spectroscopy.

Figure 4. Structural characterization of MAC@Pt. (a) XRD pattern. (b) SEM image of MAC@Pt 1000. (c) HETEM image of MAC@Pt 1000. (d) HAADF–STEM image of MAC@Pt 1000. (e) Pt 4f high-resolution XPS of MAC@Pt 1000.
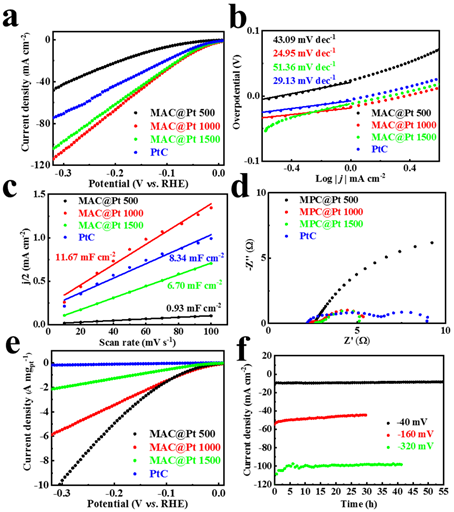
Figure 5. MAC@Pt electrochemical data. (a) LSV curve. (b) Tafel slope. (c) Cdl values. (d) Electrochemical impedance spectroscopy. (e) Mass activity. (f) Cyclic stability.
Summarize
In conclusion, the MAX-derived electrocatalyst MAC@Pt is synthesized by two-step in-situ electrochemical synthesis (electrochemical etching and electrochemical deposition). In acidic solution, MAC provides a satisfactory HER overpotential of 299 mV, outperforming most reported MXenes. A small amount of platinum nanoparticles were then anchored on the MAX-derived amorphous carbide MAC by electrochemical deposition, which further tuned the proton adsorption rate-limiting step, resulting in excellent HER performance (40 mV overpotential and 24.95 mV dec−1 Tafel slope).
Literature link
https://doi.org/10.1002/smll.202203471
For the original text, please click the lower left corner of the tweet to read the original text
















