已传文件:photo/1631586161.png
MXene is a two-dimensional star material that is even hotter than graphene. As an advanced MXene manufacturer, BeiKonami has launched special offers, 10% off mxene and other materials, and more preferential prices, waiting for you to kill! picture


Research abstract
MXenes have been studied as storage materials for alkali metal ion batteries due to their high metal conductivity, typical accordion layered structure, and abundant surface functional groups. However, as typical ion-intercalation energy storage materials, MXenes have some disadvantages, such as easy stacking of layered structure, obvious volume expansion during ion-intercalation, and slow ion diffusion rate. Especially for alkali metal ions such as Na+ and K+ with larger radii, the slow ion diffusion rate and significant volume expansion severely limit the rate capability and cycling stability of MXenes.
Introduction
Recently, Associate Professor Liu Maocheng from Lanzhou University of Technology, Dr. Liu Bao from Jiangsu University, Dr. Luo Dan from the University of Waterloo and Academician Chen Zhongwei creatively proposed the molecular welding method, which realized the controllable expansion of the interlayer spacing and the improvement of the layer structure stability of Nb2C MXene. Through the dehydration condensation between the carboxyl group of 1,3,5-benzenetricarboxylic acid (BTC) and the -NH2 group on the surface of the amino functionalized Nb2C, an amide bond is formed, and the BTC molecular welding intercalation is formed between the Nb2C layers to form the BTC molecular welding intercalation. Nb2C composite (Nb2C/BTC). Solder-intercalated BTC molecules have dual effects of pillaring and pulling on the Nb2C 2D layered structure. The pillaring effect can expand the interlayer spacing of Nb2C and prevent the reduction of the Nb2C interlayer spacing when the alkali metal ions are extracted, preventing the volume shrinkage; while the traction effect can prevent the excessive expansion of the Nb2C interlayer spacing when the alkali metal ions are embedded, preventing the volume expansion. The experimental results show that the use of molecular welding method not only expands the interlayer spacing of Nb2C, but also significantly improves its layer structure stability, thereby effectively improving the storage rate and cycle stability of alkali metal ions. The relevant results were published in Nano Energy, a top journal in the international energy field.
Research Highlights
1) A novel and effective "molecular welding" strategy is reported for the first time, which not only enlarges the interlayer spacing of Nb2C MXene, but also significantly improves the stability of its layer structure.
2) BTC molecular welding improves the Li+/Na+ storage specific capacity of Nb2C, which not only effectively accelerates the kinetic performance of alkali metal ion diffusion, but also effectively relieves the volume expansion/contraction of Nb2C during the storage process of alkali metal ions. Therefore, the alkali metal ion storage rate and cycling stability of Nb2C are significantly improved.
3) Theoretical calculations prove that BTC molecular welding can effectively reduce the migration barrier of alkali metal ions and significantly improve ion diffusion kinetics.
4) The organic molecular welding reported in this paper provides a new strategy for designing 2D energy storage materials with high rate and extraordinary cycle life.
Graphical guide
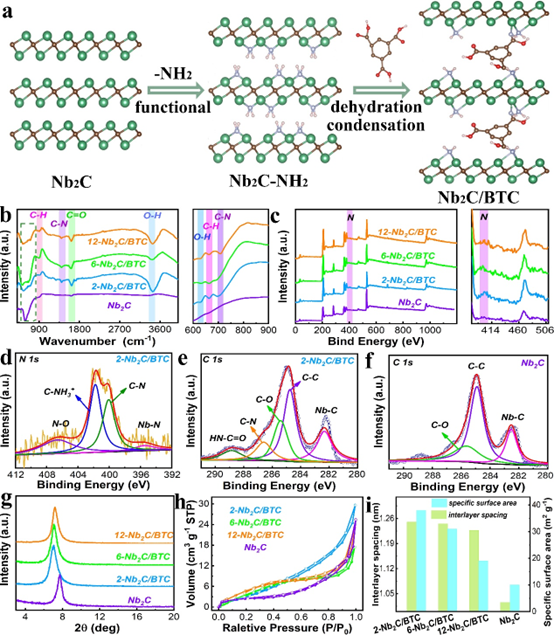
Figure 1. a) Schematic diagram of the mechanism of BTC molecular welding intercalation of Nb2C; (b) FTIR; (c) XPS; high-resolution XPS spectra of (d) N 1s and (e) C 1s of 2-Nb2C/BTC; ( f) High-resolution C 1s spectrum of Nb2C; (g) XRD; (h) N2 adsorption/desorption curves; (i) relationship between specific surface area and interlayer spacing.
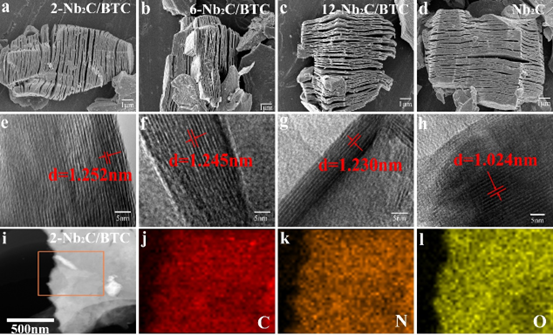
Figure 2. (a-d) SEM and (e-h) HRTEM of Nb2C/BTC and Nb2C, (i-l) TEM Mapping of 2-Nb2C/BTC.
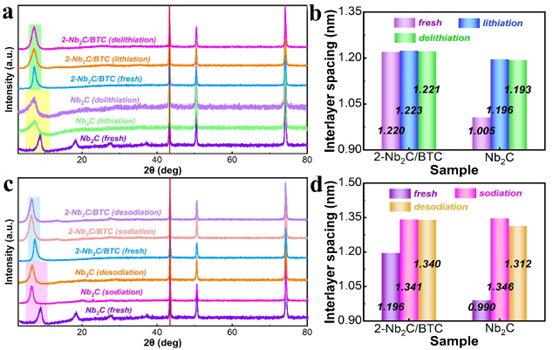
Figure 3. Ex-situ XRD patterns and interlayer spacing changes of 2-Nb2C/BTC and Nb2C in (a, b) lithium intercalation and delithiation states and (c, d) sodium intercalation and denitration states.
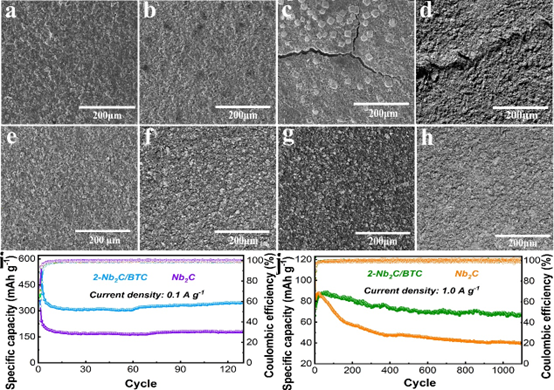
Figure 4. Ex-situ SEM of Nb2C electrodes in (a) lithiated state, (b) delithiated state, (c) sodium intercalated state and (d) de-sodium state. Ex-situ SEM images of 2-Nb2C/BTC electrode in (e) intercalated state, (f) delithiated state, (g) intercalated state and (h) deintercalated state. (i) Cyclic stability of Li+ and (j) Na+ storage.

Figure 5. Storage kinetics of Li+ ions. (a) magnification; (b) capacity retention; (c) Li+ diffusion coefficient, (d) EIS spectra of Nb2C/BTC and Nb2C. (e) EIS spectra of 2-Nb2C/BTC and (f) Nb2C after cycling in the LIBs system. (g-i) Na+ ion storage kinetics.
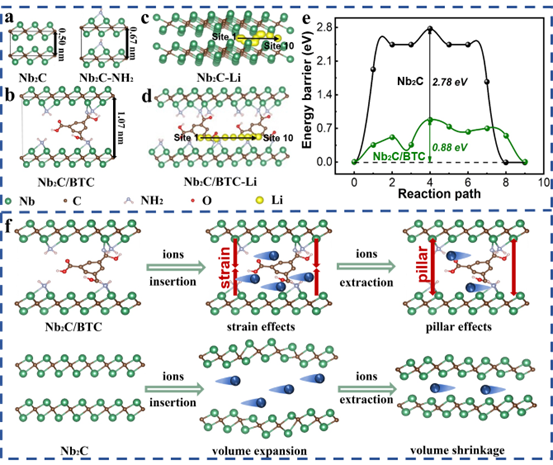
Figure 6. Theoretical calculations of the Li+ diffusion barrier for Nb2C/BTC and Nb2C. (a) Nb2C and Nb2C-NH2 side view. (b) Side view of BTC molecular welding between Nb2C layers. Diffusion paths of Li+ between (c) Nb2C and (d) Nb2C/BTC layers. (e) Li+ diffusion barrier between Nb2C and Nb2C/BTC layers. (f) Schematic diagram of the structural evolution of Nb2C/BTC and Nb2C during ion intercalation/deintercalation.
Summarize
In this study, Nb2C/BTC with stable layer structure was constructed by welding BTC molecules between Nb2C layers. The controllable expansion of the Nb2C interlayer spacing was achieved by changing the BTC molecular concentration for welding. At the same time, the two ends of the BTC molecule are welded between the Nb2C layers through amide bonds, which have dual effects of pillar support and traction on the layered structure, preventing the volume expansion/contraction of the layered structure during the insertion/extraction of alkali metal ions. Therefore, 2-Nb2C/BTC was cycled 130 times at a current density of 0.1 A g-1 to obtain a high specific capacity for Li/Na storage of 345.7/109.7 mAh g-1. In addition, 2-Nb2C/BTC exhibits high Li+/Na+ storage rate performance with a capacity retention of 61.8%/53.8% when the current density increases from 0.1 A g-1 to 1.0 A g-1. By DFT theoretical calculations, the diffusion barrier of 2-Nb2C/BTC is reduced to 0.88 eV. The proposed molecular welding method is a novel and effective method to alleviate the volume change and improve the overall storage performance of alkali metal ions.