
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online



MXene is a two-dimensional star material that is even more popular than graphene. As an advanced manufacturer of MXene, BTEC Nano has launched special offer activities, where mxene and other materials are 10% off. At the same time, BTEC Nano has launched new mesoporous material products with more preferential prices. 
The research in this paper
Mxene-based materials are widely used in energy, catalysis and other fields due to their unique geometric and electronic structure. How to understand the space environment in MXene layer is the basis of regulating the function of its ion intercalated electrode. Therefore, the intercalated electrode supported by ion column was constructed with metal ions pre-embedded in MXene, and the ion transport operation was optimized to obtain high performance charge storage. By means of electrostatic force and electrochemical drive, ions are induced to embed into the inner space of MXene layer. The spatial interaction between the preembedded ions and the MXene layer was analyzed. It was found that the preembedded metal ions could not only provide spatial support, but also modulate the functional groups on the MXene surface through ion exchange behavior, promote the ion migration of the electrolyte, and optimize the electrochemical performance of the MXene intercalated electrode. As an atomic-level modulation, ion preembedding makes it possible to accurately control the interlayer environment of low-dimensional layered materials.
Results summary
The Joint Labortory of MXene Materials, Jilin Normal University, based on the understanding of the charge storage mechanism of MXene intercalated electrodes, relying on the behavior of ion-induced spatial environment evolution in MXene layers, The intermodulation correlations between the pre-embedded ions, the space environment within the MXene layer and the function of the ion intercalated electrode were investigated.
In this work, Al3+ ions were driven to be preembedded into Ti3C2Tx MXene layer by electrochemical method, and the distribution, valence state and coordination environment of preembedded ions, crystal structure of MXene, valence state and coordination environment of constituent elements, local morphology and other related evolution information were characterized by in situ/quasi-in situ/node sampling method. Combined with the electrochemical control parameters, the migration path of the embedded ions and the evolution of the MXene in-layer confined space environment were analyzed, and the mutual modulation mechanism between the ion embedding and the in-layer limited space was revealed. It is found that electrochemical preembedding of Al3+ ions can lead to the contraction of the interlayer spacing of MXene, the occurrence of compressive strain, and the effect on the spatial charge distribution in MXene, which provides help for understanding the interaction between the interlayer charge distribution and ion insertion/ejection behavior. In addition, the performance of MXene intercalated electrode can be optimized by Al3+ ion preembedding.
The paper was Inspired by philosophizing of "Point to Area" : Ion pre - intercalation induces the reconstitution of interlayer environment of MXene for lithium storage issues published in Chemical Engineering Journal.
By reading
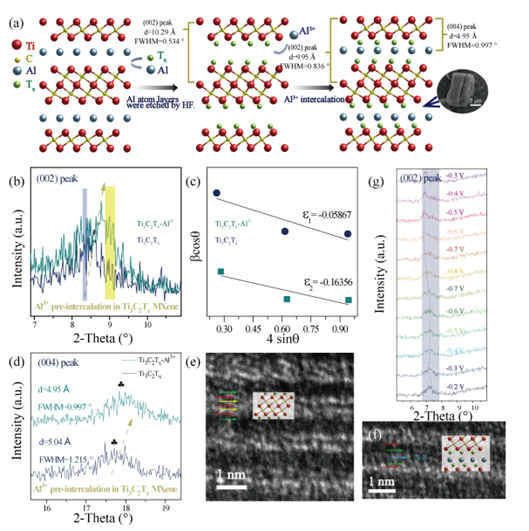
Figure 1. Schematic diagram of the preparation process of Ti3C2Tx-MXene/MWCNTs/SrFe12O19 thin films. (a) Schematic diagram of etching process of Ti3AlC2 MAX and Al3+ intercalation and SEM image of Ti3C2Tx-Al3+. (b) XRD (002) peak patterns of Ti3C2Tx MXene and Ti3C2TX-AL3 +, and (c) corresponding Williamson-Hall (W-H) analysis. (d) Magnified peak (004). HAADF-STEM images of (e) and (f) Ti3C2Tx-Al3+. (g) In situ XRD patterns of Al3+ preintercalation.
In this work, the strongly polar Al3+ ions were preembedded into Ti3C2Tx MXene using an electrochemical drive method. XRD, SEM, EDS, STEM and other test methods were used to characterize the effect of Al3+ ions pre-embedded Ti3C2Tx MXene on the layer spacing, stress, and order degree. It is found that Al3+ ions are subjected to compressive stress after preembedding, and the interlayer spacing shrinks significantly. In addition, the intercalation mechanism of Al3+ ions and the mutual modulation behavior between the pre-intercalated Al3+ ions and the MXene layer environment were systematically studied.

FIG. 2. (a) Raman spectra of Ti3C2Tx and Ti3C2TX-AL3 + MXene. In situ Raman spectra of (b) and (c) Al3+ intercalation. (d) XANES spectra; (e) FT-EXAFS spectra of Ti. (f) Ti 2p and (g) Al 2p XPS spectra. Electrostatic potential and charge density difference between (h) Ti3C2Tx and (i) Ti3C2TX-AL3 +.
In order to understand the effect of Al3+ ions pre-embedding into Ti3C2Tx MXene on the spatial environment within the layer, we monitored the intercalation process of Al3+ ions by in-situ Raman, and confirmed that with the decrease of potential, the out-of-plane compressive stress becomes larger and the interlayer spacing shrinks. XPS analysis showed that Ti gained electrons and the average oxidation state decreased significantly during ion embedding. In addition, DFT simulation was used to analyze the local environment and carrier migration behavior of MXene after Al3+ ion preintercalation. The structure-activity relationship between the optimized electrode structure and lithium storage performance was analyzed.
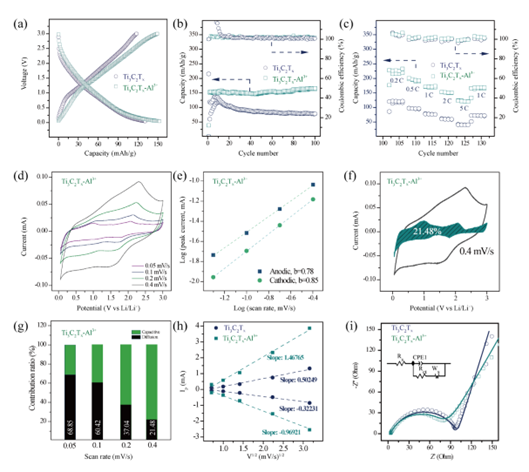
Figure 3. (a) GCD curve, (b) cycle stability, (c) magnification performance, and (d) CV curve of Ti3C2Tx and Ti3C2TX-AL3 + MXene. (e) the linearity of log(i) and log(v). (f) Capacitance contribution at a scan rate of 0.4 mV/s. (g) Percentage of capacitance contribution at different scan rates. (h) The linear relationship between peak current (Ip) of Ti3C2Tx and Ti3C2TX-AL3 + and the square root of scan rate (v1/2). (i) EIS profiles of Ti3C2Tx and Ti3C2TX-AL3 +.
Ti3C2Tx MXene preembedded with Al3+ ions showed higher Coulomb efficiency and discharge specific capacity. After 100 cycles, the reversible capacity of Ti3C2TX-AL3 + was 110.1% of the initial capacity, and the rate performance was significantly improved, which was much higher than that of Ti3C2Tx MXene. The electrochemical performance of Ti3C2Tx MXene-based lithium ion battery with Al3+ ion preembedding was improved, and the ion migration kinetics of Ti3C2Tx MXene was enhanced during lithium ion intercalation and delamination. The insertion of Al3+ ions can effectively reduce the resistance of Ti3C2Tx electrode, which is conducive to the electron contact between Li+ ions and MXene during the insertion/exit process.
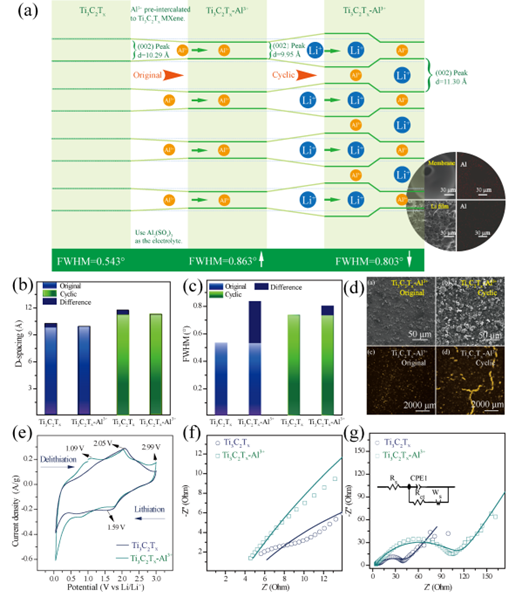
Figure 4. (a) Flow chart of Ti3C2Tx and Ti3C2TX-AL3 + electrodes after circulation. (Inset: SEM images and EDX elements of Li slices and septum). (b) layer spacing between Ti3C2Tx and Ti3C2TX-AL3 +, (c) half-height width. (d) SEM and TMM of Ti3C2Tx and Ti3C2TX-AL3 +. (e) CV curves of the third cycle of Ti3C2Tx and Ti3C2TX-AL3 +. EIS spectra after cycles in (f) and (g).
The Ti3C2Tx MXene electrode obtained a more ordered structure due to the preintercalation of Al3+. The cyclic samples were characterized by XRD, SEM, EDX, TMM and electrochemical characterization such as CV and EIS. It is found that the continuous cycling leads to the formation of cracks on the electrode surface, and the SEM images and EDX element images of lithium sheet and diaphragm after cycling show that the lithium sheet is porous, and aluminum elements exist on both lithium anode and diaphragm, indicating that part of Al3+ ions participate in the migration process. However, the pre-inserted Al3+ ions in adjacent MXene layers shrink due to electrostatic force attraction between layers, which may lead to the expansion of space in other MXene layers without pre-inserted Al3+ ions. Therefore, it can be inferred that the contribution of preintercalated Al3+ ions to stability in MXene multilayer structures may be due to the column support behavior formed by preembedding Al3+ ions in local or spacer layers.
conclusion
By electrochemically driven method, ~1% Al3+ ions were preinserted into the interlayer of adjacent Ti3C2Tx MXene. The interlayer environment, structure-function relationship and Li+ interaction between Al3+ ions and MXene in preintercalated layer are mutually modulated. Due to Coulomb effect, the MXene layer spacing shrinks at the atomic scale between the positively charged Al3+ ions and the negatively charged MXene surface. This scheme can be used as an accurate control strategy for the layer spacing d value of two-dimensional layered materials. In addition, the preembedding of Al3+ ions can induce a faster diffusion rate of Li+ ions. The electron-deficient M (Ti) layer improves the charge storage capacity, and exhibits high stability during the repeated insertion/removal of Li+ ions, which is helpful to optimize the cycling performance of MXene electrodes.


| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |