已传文件:photo/1766544328.png
Giving proteins non-natural chemical reactivity is a core strategy for expanding their biochemical functions. In recent years, protein photocatalyst conjugates have shown great potential in adjacent labeling, cell biology research, and prodrug activation due to their unique biocompatibility and high spatiotemporal resolution. However, the traditional methods for constructing such systems often rely on complex gene codon expansion techniques or non-specific chemical coupling, which face challenges such as cumbersome operation, poor site selectivity, or interference with the natural activity of proteins.
The histidine tag (HisTag) is the most widely used purification tag in recombinant protein expression, featuring a small molecular weight, minimal impact on protein properties, and broad compatibility with expression systems. Currently, the Addgene database alone contains over 9,700 plasmids containing HisTag, making it the most commonly used universal component in protein science. However, despite its extensive application, the chemical potential of HisTag has long been limited to simple physical affinity purification.
Recently, the research team led by Chen Peng and Fan Xin Yuan from Peking University published a research paper in the Journal of the American Chemical Society. This study developed a simple and universal protein photocatalytic enabling strategy - CAT-tag. This technology ingeniously utilizes the most common HisTag in protein engineering as a metal ligand, and performs in-situ coordination assembly with the inactive iridium catalyst precursor. It achieves a functional transition from "purity tag" to "photocatalytic center" in one step, endowing proteins with efficient photocatalytic activity. At the same time, CAT-tag technology is also the latest member of their "CAT-X" technology system, jointly constructing a complete biotransformational photocatalysis technical matrix, providing a powerful chemical tool for the spatiotemporal analysis of the photocatalysis in biological processes. 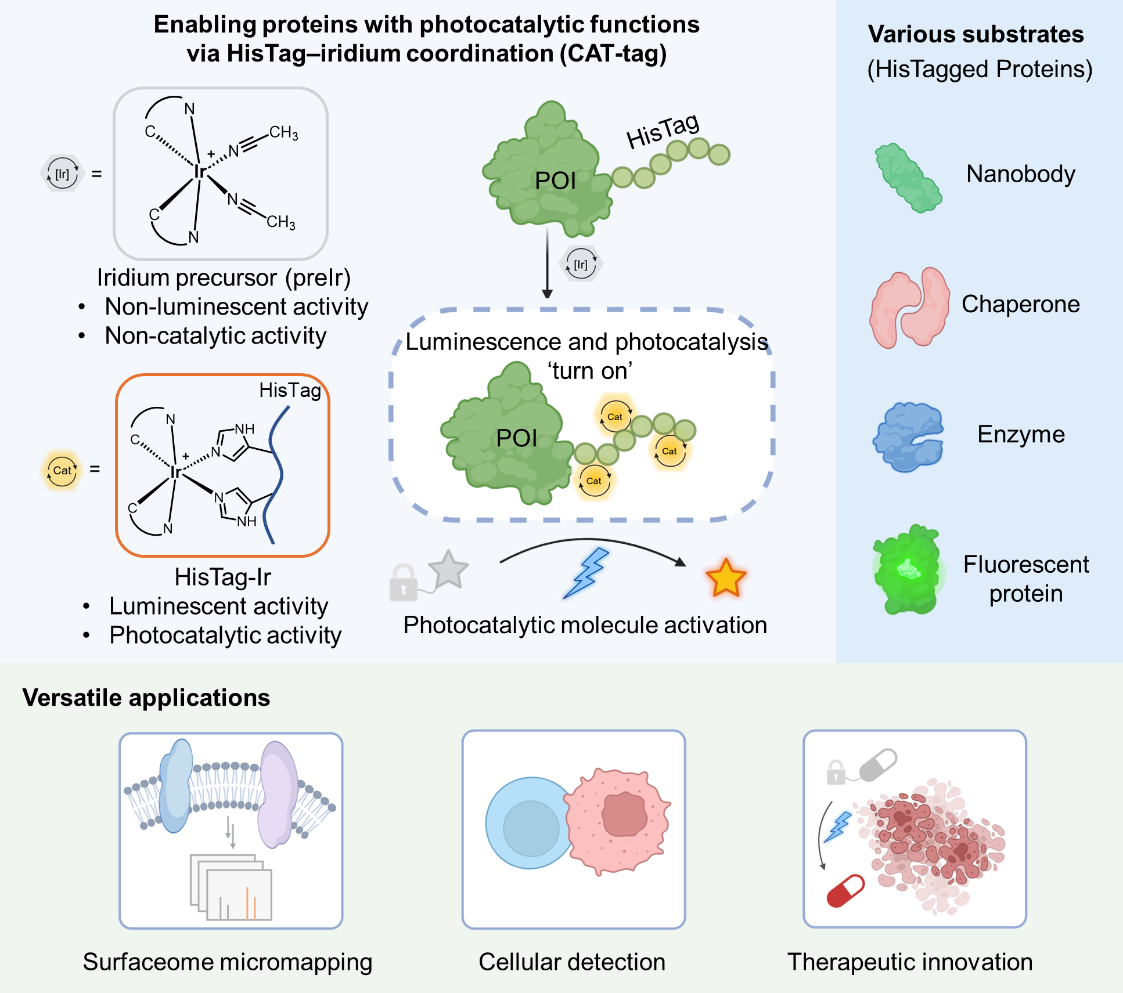
Figure 1. The principle and application of CAT-tag technology
1. Scientific Design: From "Affinity" to "Catalysis" in Chemical Design
HisTag (typically 6×His) is rich in imidazole groups. Inspired by the structure of the bipyridine (bpy) ligand in iridium catalysts, the authors utilized the coordination properties of the imidazole group of histidine, which are similar to those of bipyridine (sp2 hybridized nitrogen atom), to propose the hypothesis that HisTag can be used as a multi-dentate ligand to directly anchor the iridium metal center. To achieve biocompatibility and controllable reactions, the authors designed and screened a series of iridium complex precursors coordinated with acetonitrile. Among them, preIr4 with carboxyl group modification exhibited the best water solubility and reactivity. PreIr4 showed good stability in a pure aqueous phase and had no photocatalytic activity or fluorescence signal, effectively avoiding background interference. When preIr4 coordinated with the imidazole group of histidine to form the Ir(Im)2 structure, its photophysical properties underwent a significant transition, showing a maximum emission wavelength of 540 nm and strong fluorescence with an excitation energy of up to 2.48 V. In 13 representative amino acid competition tests, preIr4 showed highly specific coordination selectivity for histidine. Combined with kinetic studies, it was demonstrated that the standard 6×His tag could simultaneously coordinate three iridium complex units, forming a stable HisTag-Ir3 catalytic center.
2. Universal verification: Plug-and-play photocatalytic module
Subsequently, the author verified the universality of the CAT-tag strategy on various types of HisTag fusion proteins, covering different scaffolds such as affinity bodies, nanobodies, enzymes, and fluorescent proteins. These proteins only needed to undergo the standard HisTag purification steps and could directly react with preIr4 in PBS buffer (at 37°C) for 1 hour, quickly transforming into protein-phosphoromaterial conjugates with fluorescence and photocatalytic activity. It is noteworthy that even the FDA-approved bispecific antibody drug Blinatumomab can be simply converted into a photocatalytic system through this strategy. 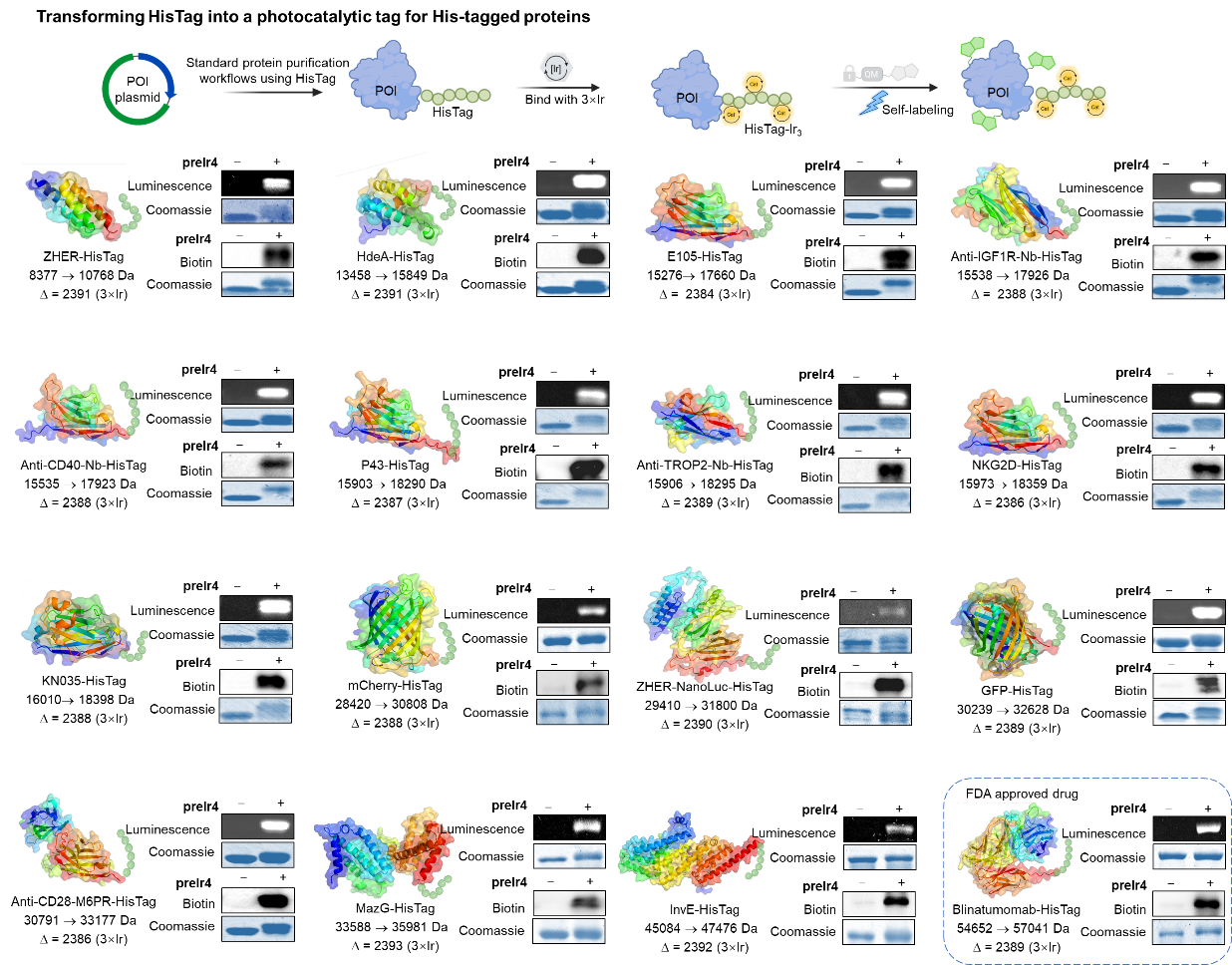
Figure 2. Generality test of the CAT-tag technology.
3. Application Scenario: Spatiotemporal Analysis and Intervention in Three Biological Dimensions
The author demonstrated the potential of CAT-tag in three biological dimensions:
"Micro-region" mapping of cell surfaceomics: Drawing on the previously developed CAT-Ex technology (Chem 2022, 8, 2179), using the anti-HER2 nanobody photocatalyst (ZHER-HisTag-Ir3) constructed with CAT-tag, under blue light excitation, the thiosemicarbazone probe SF2 was activated through a bioorthogonal reaction, generating highly active intermediates. This system successfully performed precise biotinylation labeling of the adjacent microenvironment on the surface of HER2-positive cells and identified various functional membrane proteins including ion channels and transport proteins through high-resolution mass spectrometry, revealing the "social circle" of the HER2 receptor on the membrane surface.
(2) Capturing dynamic cell interactions: Drawing on the previously developed CAT-Cell and CAT-Tissue technologies (J. Am. Chem. Soc. 2024, 146, 15186; J. Am. Chem. Soc. 2025, 147, 9716), for transient cell communication processes, the authors constructed CAT-tag photocatalytic probes using anti-PD-L1 nanobodies. In the cell interaction model mediated by CD40-CD40L, this system can selectively label "prey" cells that interact with the "trapping" cells, while not non-specifically labeling cells that do not interact. This result demonstrates the high signal-to-noise ratio advantage of the CAT-tag system in analyzing complex cell communication networks.
(3) Precise prodrug activation with controllable time and space: Drawing on the previously developed CAT-NIR technology (Angew. Chem. Int. Ed. 2023, 62, e202310920), the CAT-tag can also endow antibody drugs with a new function of "light-controlled killing". The authors synthesized a cytotoxic prodrug (PAB-MMAE), which is non-toxic when existing alone, but can be specifically released with high activity drug MMAE when anchored on the surface of tumor cells and catalytically activated by ZHER-HisTag-Ir3. Experimental data show that this strategy achieved specific killing of HER2-positive tumor cells under light conditions, significantly reducing off-target toxicity to normal cells.
In summary, through ingenious coordination chemistry design, the CAT-tag technology has redefined the functional boundaries of HisTag, upgrading it from a simple purification tag to a programmable photocatalytic module. This strategy has the significant advantages of "one-step assembly, site-specificity, and high universality", providing a new chemical paradigm for the design of artificial metal enzymes and offering powerful chemical-biological tools for spatiotemporal resolution biological research and precision medicine.
Professors Fan Xinyuan and Chen Peng from the School of Chemistry and Molecular Engineering at Peking University are the corresponding authors of this paper. Postgraduate student Guo Haotian and postdoctoral researcher Liu Ziqi are the co-first authors of this paper. This research work was supported by funds from the National Natural Science Foundation of China, the National Key Research and Development Program of the Ministry of Science and Technology, and the Beijing Natural Science Foundation, among others.





