
【Research Background】
NH3 is the most common alternative to chlorofluorocarbons (CFCs) in refrigeration systems. It is a toxic compound that is highly volatile and extremely harmful to humans. On the other hand, NH3 is widely used in the chemical industry to synthesize various materials, and is expected to become the future automotive energy carrier. NH3 is also expected to serve as an NH3 repository for selective catalytic reduction (SCR) of nitrogen oxides for diesel vehicles and trucks. Therefore, it is necessary to find an effective method to detect and capture NH3 gas for atmospheric environmental control and industrial applications. Semiconductor metal oxides and low-dimensional materials are considered to be the most promising candidate for gas sensors because of their low cost, small size, and good compatibility with traditional microelectronic machining. However, the main disadvantage of these sensors is the lack of selectivity for a particular gas (NH3), and since NH3 tends to interact strongly with a variety of substrates, most sensors recover longer at room temperature.
Among all MXenes, Ti2C is one of the thinnest. During the etching with HF acid, the surface of Ti2C will have F-based, OH-based and O-based functional groups. It has been found that the electronic properties of Ti2C have a strong dependence on functional groups. Only Ti2CO2 exhibits semiconducting properties, and Ti2C, Ti2CF2 and Ti2C(OH)2 exhibit metallic properties. Therefore, Ti2CO2 may have more potential applications due to its semiconductor properties.
[Introduction]
Associate Professor Xiao Bo of Yantai University explored the potential of single-layer Ti2CO2 as a gas sensor and capture through first-principles simulation. Compared with other gas molecules (H2, CH4, CO, CO2, N2, NO2 and O2), the preferential adsorption of NH3 on a single layer of Ti2CO2 indicates that the single layer of Ti2CO2 has excellent selectivity to NH3. The I-V relationship before and after NH3 adsorption changed significantly, indicating that the single layer Ti2CO2 has strong sensitivity to NH3. More importantly, the interaction of the monolayer of Ti2CO2 with NH3 can be easily adjusted by strain, which indicates that it is easy to capture and release NH3 using a monolayer of Ti2CO2.
This work was published online at ACS Applied Materials & Interfaces: Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity.
[Graphic introduction]
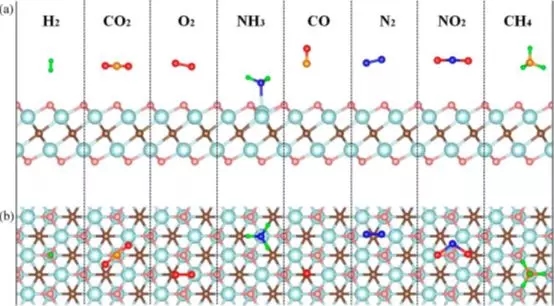
Figure 1. Side view (a) and top view (b) of the molecular structure of a single layer of Ti2CO2 adsorbing NH3, H2, CH4, CO, CO2, N2, NO2 or O2 molecules.
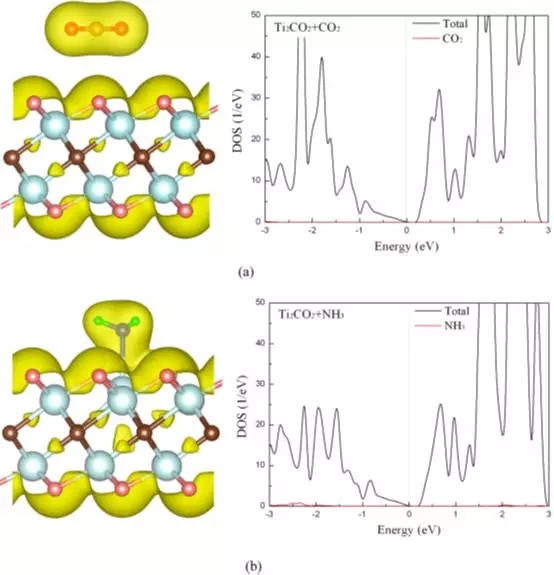
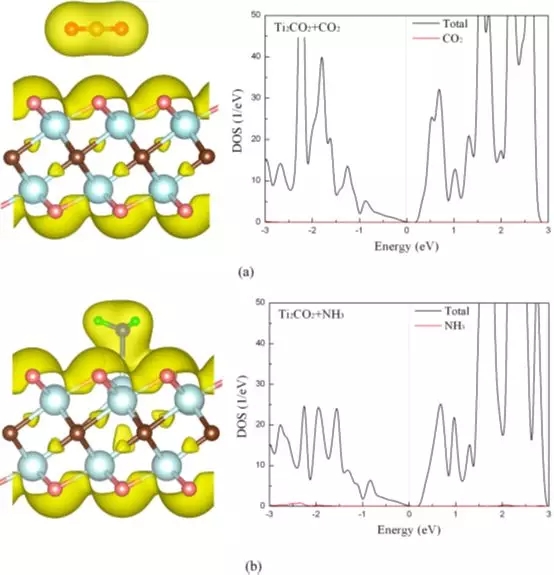
Figure 2 Total charge density and density of states of CO2 (a) and NH3 (b) adsorbed on a single layer of Ti2CO2.
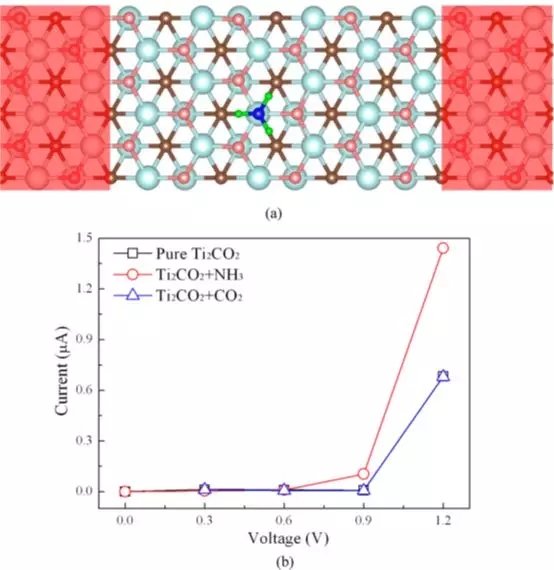
Figure 3 (a) Schematic diagram of the detection of NH3 molecules based on Ti2CO2 sensors; (b) Current-voltage (I - V) relationship before and after adsorption of NH3 or CO2 molecules on a single layer of Ti2CO2.
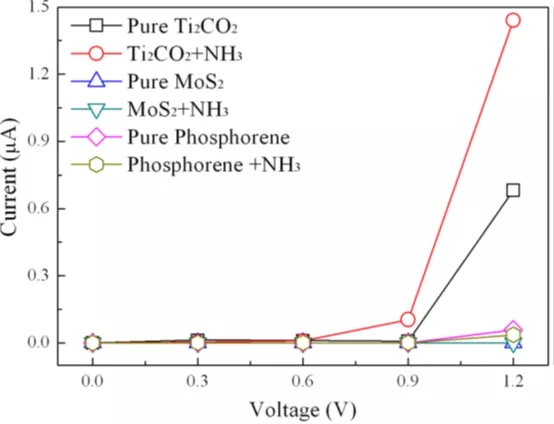
Figure 4 shows the I-V curve of the calculated NH3 before and after adsorption on Ti2CO2, MoS2 and phosphonene.
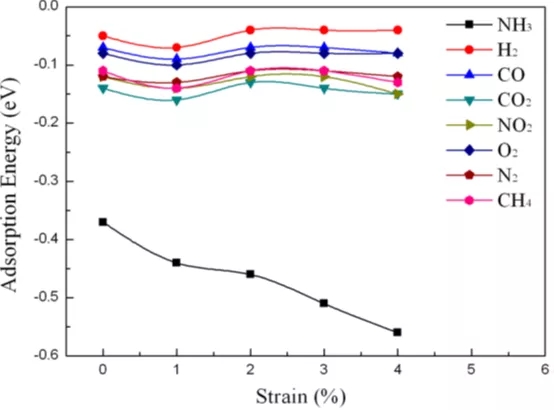
Figure 5 The adsorption of gas molecules (including NH3, H2, CH4, CO, CO2, N2, NO2 or O2) on a single layer of Ti2CO2 as a function of the applied biaxial strain (from 0 to 4%).
[Summary of this article]
Through first-principles simulation, this paper proves that Ti2CO2 is expected to be a NH3 sensor with high sensitivity and selectivity, mainly for the following reasons: (1) with other gas molecules (H2, CH4, CO, CO2, N2, NO2 and Compared with O2), only NH3 can be chemisorbed on a single layer of Ti2CO2; (2) NH3 has an adsorption energy of -0.37 eV on Ti2CO2, which is suitable for the adsorption/desorption of gas on a solid surface. Therefore, after detecting NH3, Ti2CO2 The sensor can be easily recovered; (3) After the adsorption of NH3, the conductivity of Ti2CO2 is significantly improved, resulting in high sensitivity of the Ti2CO2 sensor to NH3. In addition, the strain applied on Ti2CO2 can further enhance the interaction between NH3 and Ti2CO2, that is, when the strain is 3%, the adsorption energy is -0.51 eV, and under the same strain, the adsorption of other gases on Ti2CO2 is better than that of NH3. Much weaker. Therefore, the results show that Ti2CO2 can be used as a capture, separation and storage material for NH3 by controlling strain.
Literature link
Https://doi.org/10.1021/acsami.5b03737
Source: WeChat public number MXene FrontierFigure










