
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online


【Research Background】
Ammonia (NH3) is widely used in various fields, including nitrogen fertilizers, industrial refrigerants, biofuels and textiles. However, it is one of the most harmful environmental pollutants produced in general industrial production and manufacturing processes. The explosion limit of ammonia is 16-25%. According to the standards published by the U.S. Occupational Safety and Health Administration, the acceptable ammonia concentration in the human body is limited to 8 hours at 25 ppm or 15 minutes at 35 ppm. If humans are exposed to large amounts of ammonia, the skin, eyes, or respiratory system can be affected, leading to blindness and lung disease. At the same time, the presence of excess ammonia in human breath may be related to human diseases. Therefore, finding high-performance sensors capable of detecting ammonia is of great significance to production safety and human health.
Over the past few decades, various materials have been developed for detecting toxic gases, including metal oxide semiconductors and carbon nanomaterials. However, most of them are tested at high temperatures, which makes them not only energy-intensive but also unsuitable for daily life. MXene has a high specific surface area due to its 2D structure, and its surface is rich in functional groups. The conductivity of MXene can be changed by the type and number of surface functional groups. So in theory, MXene can be used as a gas sensor. In recent years, there have been some reports of Ti3C2 used in gas sensors, but no experimental research has focused on NH3 detection.
[Achievement Profile]
In 2019, Professor Zhu Zhigang of Shanghai Second Polytechnic University and Professor Zhou Aiguo of Henan University of Technology reported the experimental research and theoretical calculations of NH3 sensing of Ti3C2 MXene. In the literature, they used Ti3C2 (with a clean surface) made by NaF + HCl etching to test its gas-sensitive properties. Experimental measurements show that a single layer of Ti3C2 MXene can be used to construct a highly selective NH3 sensor at room temperature. The theoretical calculation supports the high selectivity of Ti3C2 MXene for NH3. The effects of sensor structure, humidity, and NH3 concentration are studied in this article. It is found that Ti3C2 MXene is an ideal candidate sensing material for detecting NH3 at low temperature. This work laid the foundation for the application of Ti3C2 sensors in the field of flexible electronic monitoring.
The results were published online in ACS Sensors: Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature
[Picture and text guide]
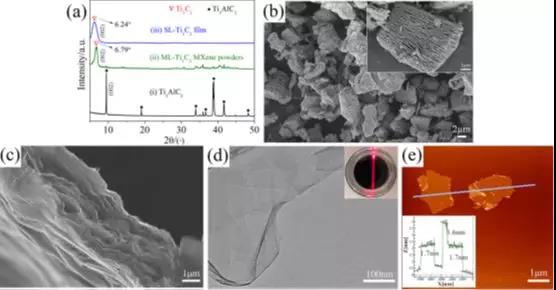
Figure 1 Ti3C2 microstructure: (a) (i) Ti3AlC2, (ii) ML-Ti3C2MXene powder, and (iii) XRD pattern of SL-Ti3C2 film. (b) SEM image of ML-Ti3C2 MXene powder. (c) SEM image of a cross-sectional view of the SL-Ti3C2 film. (d) TEM image of SL-Ti3C2 nanosheets. Inside is the Tyndall effect of SL-Ti3C2 suspension. (e) AFM image of SL-Ti3C2 nanosheets.
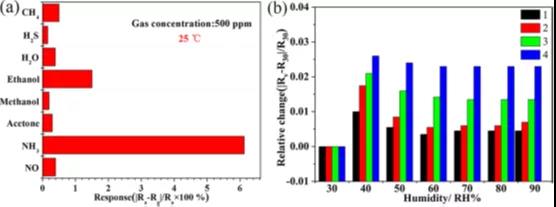
Figure 2 Ti3C2 sensor response at room temperature. (a) Gas sensing results of Ti3C2 MXene sensor for methane, hydrogen sulfide, water, ethanol, methanol, acetone, ammonia and nitric oxide at room temperature (25 ° C). (b) Effect of ambient humidity on the relative resistance change of Ti3C2 sensors with different layers of sensitive films.
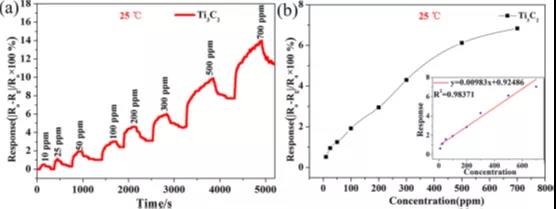
Figure 3 (a) Dynamic response of Ti3C2 gas sensor to different concentrations of NH3 at room temperature. (b) The relationship between the response of the Ti3C2 gas sensor and the NH3 concentration at room temperature.
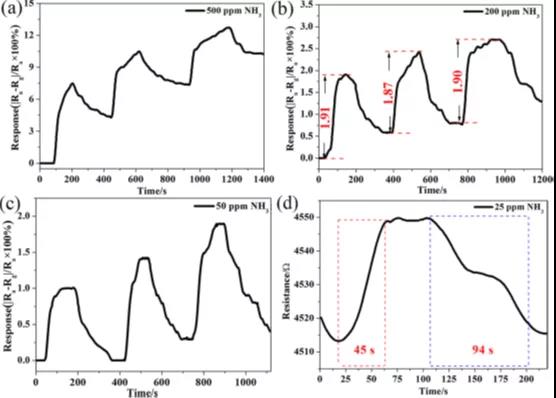
Figure 4 The cyclic response of the Ti3C2 sensor to 500 ppm ammonia (a), 200 ppm ammonia (b), and 50 ppm ammonia (c). (d) Response-recovery curve for 25 ppm ammonia.
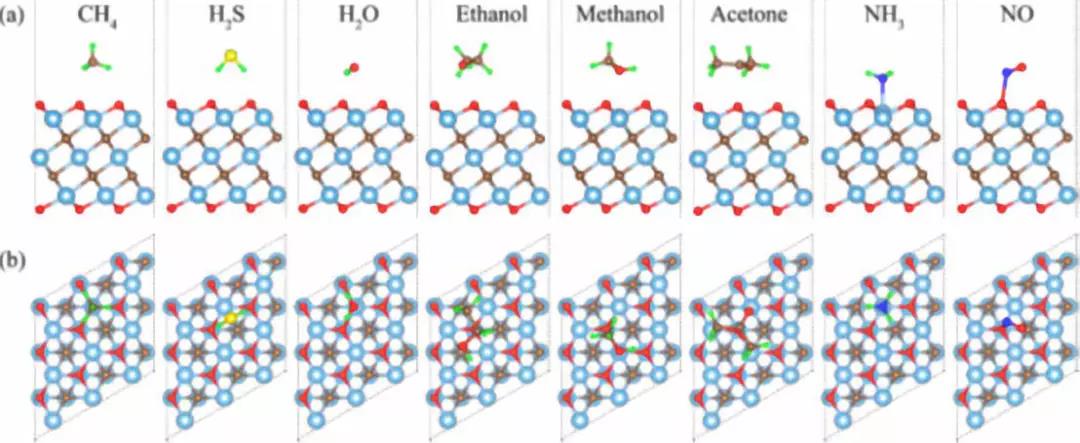
Fig. 5 Side view (a) and top view (b) of the most stable configurations of different gas molecules on the surface of Ti3C2O2.
[Summary of this article]
The Ti3C2 sensor in this paper is composed of a single layer of Ti3C2 MXene suspension. Theoretical and experimental studies show that the sensor has good NH3 detection performance at room temperature.
By inserting and delaminating ML-Ti3C2, SL-Ti3C2MXene suspension was successfully synthesized. The suspension was coated on a ceramic tube, and a Ti3C2 gas sensor was constructed. The sensor has a resistive response to eight gases (methane, hydrogen sulfide, water, ethanol, methanol, acetone, ammonia, nitric oxide). Among the eight gases, the sensor has good selectivity for NH3, both large and small molecules. The response to NH3 is 6.13%, which is four times the second highest response (1.5% response to ethanol). According to theoretical calculations, the selectivity of NH3 is caused by the compression effect of adsorption energy, adsorption geometry and charge transfer. In addition, a series of tests were conducted to study the performance of the sensor in real-world applications. The results show that the electron tube coated with a single layer is the best sensor, and the sensor is less affected by humidity. A series of tests were performed on ammonia, and the results showed that the prepared Ti3C2 sensor had good repeatability for ammonia detection at room temperature, with a minimum detection concentration of 10 ppm. Therefore, Ti3C2 MXene is an ideal candidate sensing material for room temperature NH3 detection, and can be extended to applications such as flexible electronics and instant care.
Literature link:
https://doi.org/10.1021/acssensors.9b01308

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |