
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

[Background introduction]
MXene, as an important two-dimensional nanomaterial, has received extensive attention in many fields due to its unique physical and chemical properties. At present, most preparation methods mainly rely on water as a main solvent for selective etching, and the intercalation water introduced during the preparation process, -O / -OH functional groups. The presence of intercalation water and -O / -OH functional groups greatly limits the application of MXene in nanocomposites and electrode materials for energy storage devices. More importantly, they greatly reduce the stability of MXene.
In order to solve the above problems, Michel W. Barsoum‘s group at Drexel University in the United States has developed a method for preparing MXene with high fluorine content by etching and stripping in a polar solvent. Different from most previous reports, this method achieves selective etching of Ti3AlC2 by NH4HF2 in different organic solvent systems, and prepares high-fluorine intercalated Ti3C2Tz MXene. At the same time, the Ti3C2Tz MXene prepared by this method has better performance in the storage of sodium ions than the Ti3C2Tz MXene prepared by the traditional aqueous phase etching method. The working day was published in the famous international journal Chem under the title "2D Ti3C2Tz MXene Synthesized by Water-free Etching of Ti3AlC2 in Polar Organic Solvents".
[Picture and text guide]

Figure 1. Schematic of the etching and washing process using a polar organic solvent polycarbonate (PC) as an example. A. Ti3AlC2 precursor, B. After etching with NH4HF2, C. After washing with HCl / isopropanol, D Schematic diagram of the product after suction filtration
Figure 2. Comparison of XRD spectra of Ti3C2Tz MXene prepared in different organic solvent systems: A. The etched Ti3C2Tz MXene, B. The spectrum in the 1.2 ° -10 ° range in Figure A shows the 002 peak. Comparison of the XRD spectra of the samples after washing, drying, filtering and forming the films of Ti3C2Tz MXene prepared in the organic solvent system
The article explores the etching products of different organic solvent systems. As shown in Figure 1, when etching in a PC system, NH4 + forms complexes with polar molecules and intercalates between MXene sheets. It can be seen from FIG. 2A that Al salts such as AlF3 and (NH4) 3AlF6 are generated during the etching process. Considering that the polar solvent used in the test has low solubility to the Al salt, it is necessary to use HCl / isopropanol mixed solution for MXene Wash to achieve the purpose of salt removal (Figure 1 C). The comparison of XRD spectra before and after washing showed that the 002 peak was significantly shifted, which indicates that the interlayer distance has changed, which may be due to the ion exchange between NH4 + and H + during the washing process. It is worth noting that under the DMSO system, the interlayer distance did not change before and after washing, indicating that ion exchange occurred under this system. Compared with the multilayer MXene prepared by HF etching, the distance between the MXene layers prepared by the organic solvent system is increased by about 0.45 nm, which is due to the intercalation of the organic solvent molecules.

Figure 3. A. SEM image of multilayer PC-MX, B. TEM image of PX-MX after peeling, C. B. selected area diffraction (SAED) spots, D. digital photo of PC-MX dispersion
By scanning electron microscope and transmission electron microscope to characterize the morphology of the product, it can be seen that the etched multilayer MXene also has a typical accordion-like multilayer structure. After stripping, TEM photos and selective diffraction did not find the presence of TiO2. This is also the aspect of this method due to aqueous etching.

Figure 4. A. XPS spectrum of PX-MX after etching and washing, B. XPS spectrum of PX-MX after 12h exposure to external environment
Among the PC-MX functional groups after washing, -F accounts for about 70%, while -O / -OH accounts for about 30%. This part of the oxygen-containing functional groups may be due to -F in the air during short exposure during the washing Replaced by –O / –OH. In order to further verify the conjecture, the experimenter performed XPS characterization after exposing the sample in Fig. 4A to the air for 12 hours, and found that the proportion of F atoms was only 0.07, which further proved the previous conjecture.

Figure 5. The electrochemical performance of PC-MX as the negative electrode of sodium ion battery A. Cyclic voltammetry curve for the first 10 cycles when the sweep speed is 0.2 mV s-1, B. The cycling performance at 100 mA g-1 Rate performance test and coulomb efficiency at 100, 200, 500, and 1,000 mA g-1
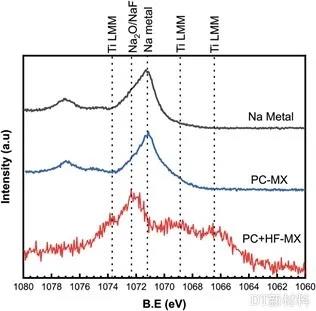
Figure 6. Comparison of Na 1s spectra of Na foil, PC-MX coated Na foil, and PC + MX-HF coated Na foil
In order to investigate the electrochemical performance of MXene prepared by this method, PC-MX was selected as the electrode material of sodium ion batteries. Cyclic voltammetry tests showed that the intercalation behavior of Na ions in PC-MX was consistent with existing reports. At the same time, it was proved that PC-MX has good electrochemical performance through cyclic charge and discharge test and rate performance test.
In order to investigate the application of MXene prepared by organic solvent systems under water sensitive conditions, the researchers designed corresponding comparative experiments. Researchers prepared MXene in an HF aqueous solution, dried it and dispersed it in a PC solvent, which was recorded as PC + MX-HF. PC-MX and PC + MX-HF were dispersed in PC and coated on the surface of Na foil, and then XPS tests were performed on different Na foils. Both Na-O and NaF were detected in PC-MX-coated Na foil and Na foil without any treatment, while PC + MX-HF-coated Na foil was detected. This comparative experiment fully demonstrates that the MXene prepared under the organic solvent system is more conducive to the application in water-sensitive environments.
[Research Summary]
In this work, researchers successfully prepared Ti3C2Tz MXene with high fluorine content by using NH4HF2 as an etchant in a variety of organic solvents, which is beneficial to explore the physical and chemical properties of Ti3C2Tz MXene based on high fluorine content. At the same time, the use of a variety of polar organic solvents is also conducive to the industrial preparation of MXene. At the same time, the article shows the good application prospect of Ti3C2Tz MXene prepared in PC solvent system in the field of sodium ion storage. The idea of preparing MXene by etching in an organic solvent system carried out in this work has greatly expanded the application of MXene in an environment that is extremely sensitive to water.
references
1. Natu et al., 2D Ti3C2Tz MXene Synthesized by Water-free Etching of Ti3AlC2 in Polar Organic Solvents, Chem (2020), https://doi.org/10.1016/j.chempr.2020.01.019
2. Zhao et al., Synthesizing MXene Nanosheets by Water-free Etching, Chem (2020), DOI: 10.1016 / j.chempr. 2020.02.013

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |