Porous bismuth-carbon composite nanoparticles help achieve fast response and stable nickel-bismuth batteries
In practical applications, the use of renewable natural energy such as solar energy, wind energy, and tidal energy will be limited and affected by factors such as time, place, and climate. The best way to use it is to efficiently convert it into electrical energy and immediately store it in a dedicated battery device. Water-based secondary batteries have significant advantages in terms of power performance, operation and maintenance costs, and safety assurance, so they have broad application prospects in the field of large-scale power grid energy storage. Metal bismuth (Bi) is considered as one of the negative electrode materials for rechargeable aqueous batteries due to its large volume capacity (~ 3765 mAh cm-3), suitable working potential window, and highly reversible redox reaction. However, the electrochemical stability of Bi-based electrode materials is poor, which makes the design and development of full-cells very slow. To make matters worse, Bi-based materials (such as bismuth oxide, etc.) are easy to decompose into elemental materials after heating, and the melting point of elementary Bi is relatively low (only about 271 ° C), and the resulting products are prone to "melt aggregation". Therefore, it is difficult to achieve a structure-controllable Bi-based composite material (especially a nanomaterial) through traditional inexpensive electrode interface optimization techniques (such as carbon modification) to alleviate the problems of battery specific capacity attenuation and poor stability.
Recently, Jiang Jian‘s group from the School of Materials and Energy of Southwest University proposed a more unique electrode material processing scheme, that is, using NH₄Bi₃F ₀ nanospheres as precursors, and successfully obtained hollow Bi simple nanoparticle @ carbon composite material after high temperature treatment. It is worth emphasizing that after heating at high temperature, its nano-scale structure remains almost unchanged and no fusion occurs. We believe that the high heat resistance (annealing temperature increased to 400oC) exhibited during the processing of this material is closely related to the high binding energy of the Bi-F chemical bond. This Bi @ carbon composite has a unique hollow nanostructure, has a high specific surface area, unobstructed ion transmission channels, and strong mechanical stability. When used as a negative electrode material, it can exhibit high specific capacity and rate characteristics. (53.9% capacity retention rate at high current density of 20 A g-1), and long cycle life (capacity retention rate exceeds 80% after 1000 cycles). More importantly, through the in-situ characterization (such as XRD, Raman) analysis of the electrode at different cycle stages, we further confirmed the unique reversible phase transition process of the anode, namely "Bi ↔ Bi (OH) 3", the assembled Ni- Bi full battery has excellent energy / power density, fast response capability and sufficiently stable electrochemical performance, which is suitable for fast collection and storage of electricity generated by new energy.

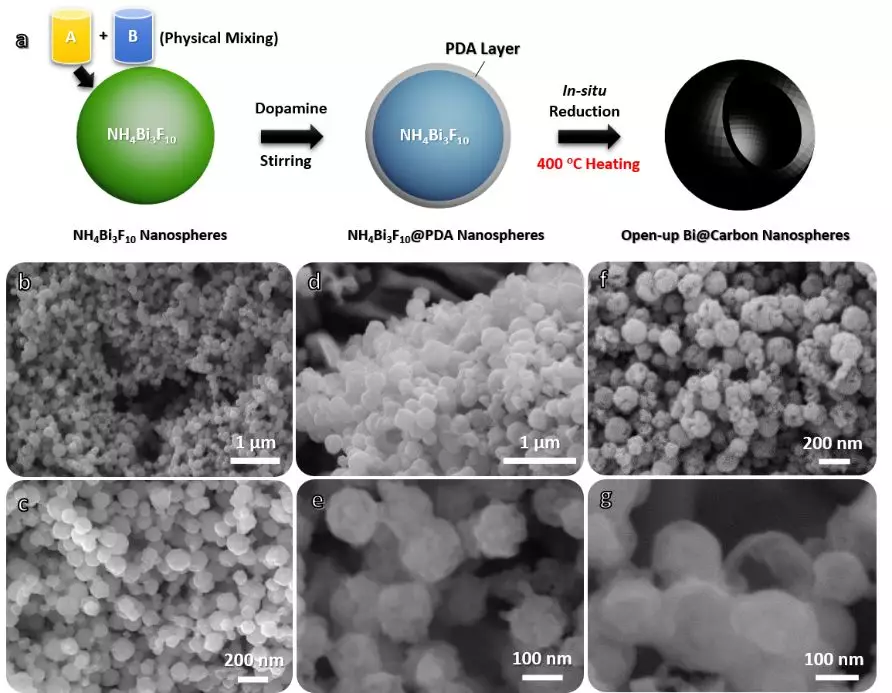
Figure 1 Flow chart of Bi @ carbon composite preparation and SEM photos of samples at different evolutionary stages
As shown in Figure 1a, the overall manufacturing process of the composite includes three steps. First, the initial material of NH4Bi3F10 nanospheres was obtained by the traditional liquid phase method. Specifically, the NH4F and Bi (NO3) 3 ethylene glycol solution can be physically mixed to instantly react to form a uniform NH4Bi3F10 nanosphere (50 nm). Next, dopamine was applied to the NH4Bi3F10 nanoparticles in situ (thickness: 5-10 nm) to generate the intermediate NH4Bi3F10 @ PDA. Finally, the intermediate product is annealed in a nitrogen atmosphere. The chemical equations involved are as follows:
10NH4F + 3Bi (NO3) 3 → NH4Bi3F10 + 9NH4NO3
NH4Bi3F10 → NH4F + 3BiF3
xBiF3 + 3C → xBi + 3CFx (x = 1 ~ 4)
From the SEM tracking observations (Figure 1b-g), it can be seen that the sample is always highly dispersed and maintains a spherical profile without particle distortion and aggregation. In particular, each Bi @ carbon nanoparticle exhibits an open state of mesopores, and its formation may be due to the release of NH3 and CFx by the material decomposition during annealing.
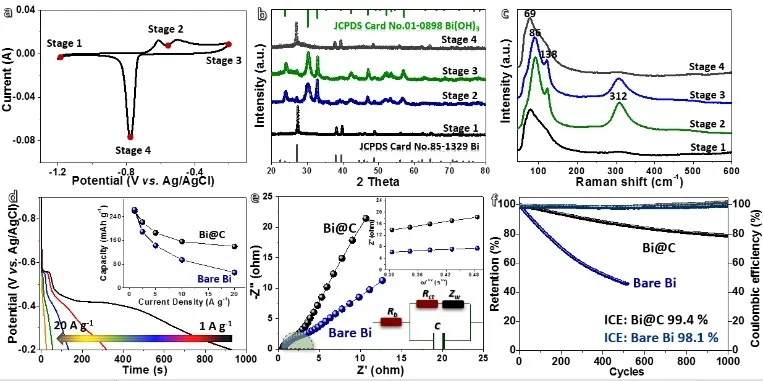
Figure 2 Electrochemical performance test results of Bi @ carbon composite nanoparticles.
Figure 2 shows the electrochemical performance test results of Bi @ carbon composite nanoparticles. In order to understand the real-time chemical changes of Bi @ carbon, we used in situ XRD to detect its phase evolution at different CV scanning stages (Figure 2b). According to XRD pattern analysis, it can be known that the original metal Bi (stage 1) is directly oxidized to Bi (OH) 3 (stage 2), instead of other possible Bi +, Bi2 + and Bi3 + (such as BiO, Bi (OH) 2, Bi2O3, etc.). This detection result is different from that of a micrometer-sized Bi electrode (Bi↔Bi2O3). In addition, almost no diffraction signal of metastable Bi + was detected. We think this is caused by the too fast reaction rate of nano-Bi @ carbon. The in-situ Raman test (Figure 2c) analysis results are consistent with the previous XRD results, thereby confirming the reversible phase transition of Bi0 and Bi3 + during the charge and discharge process. Based on the above discussion, the reversible electrochemical reactions involved can be described as:
Bi + 3OH--3e- ↔ Bi (OH) 3
The constant current discharge curves at different current densities (Figure 2d) show that the capacity of the Bi @ carbon electrode at high current density is significantly higher than that of pure Bi nanoparticles.
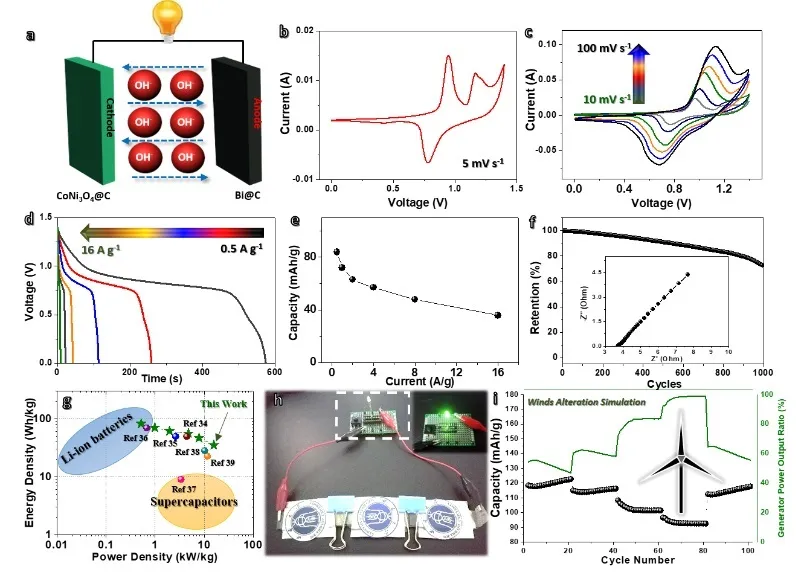
Figure 3 Full cell performance test results based on Bi @ carbon composite nanoparticle electrodes.
Figure 3 shows the results of a full cell performance test based on a Bi @ carbon composite nanoparticle electrode. The test results show that the fast-responding Bi @ carbon composite nanoparticles have potential application prospects. Our work not only provides an ingenious synthesis method for the realization of Bi-based water-based batteries, but also proves their unique reversible phase change process and fast response characteristics, and provides the rapid collection and storage of electricity for new energy generation reference.
Fast-response / stable Ni-Bi cells achieved by open-up Bi @ carbon nanospheres: a preferred electricity collection choice to couple with clean energy harvesting
Jiajia Yao, Linpo Li, Ning Li, Jian Jiang * Yanlong Wang, and Jianhui Zhu *
Mater. Chem. Front., 2020, Accepted Manuscript
http://dx.doi.org/10.1039/D0QM00017E
Frontiers Journals







