
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

 【introduction】
【introduction】
With people‘s dependence on lithium-ion batteries in daily life, people‘s attention to the safety of lithium-ion batteries has grown rapidly. Among them, highly flammable organic electrolytes bear most of the safety responsibility. A safer aqueous electrolyte instead of an organic electrolyte will greatly improve battery safety, but the narrow electrochemical stability window of the aqueous electrolyte severely limits the energy density of the aqueous lithium-ion battery. In order to solve this problem, it is necessary to reduce the water molecule content and electrochemical activity in the Li + solvation structure of the aqueous electrolyte. Recently, a new water-based electrolyte, water-in-salt (WiS) electrolyte, has increased the electrochemical stability window of the water-based electrolyte to about 3.0 V. This is because the average number of water molecules in each Li + solvated structure is much lower than the number of water molecules in Li + solvated structures in ordinary dilute solution electrolytes. Li + solvation structure and electrolyte Bulk structure have undergone significant changes, leading to changes in the interface chemical reaction of the electrolyte on the electrode surface, thereby widening the stable electrochemical window of the aqueous electrolyte. In order to further broaden the stable electrochemical window of WIS electrolyte, the lithium salt concentration can be further increased. However, the further increase of the lithium salt concentration is usually limited by the solubility of the lithium salt, and the accompanying increase in viscosity and ion conductivity Low problem.
[Achievement Profile]
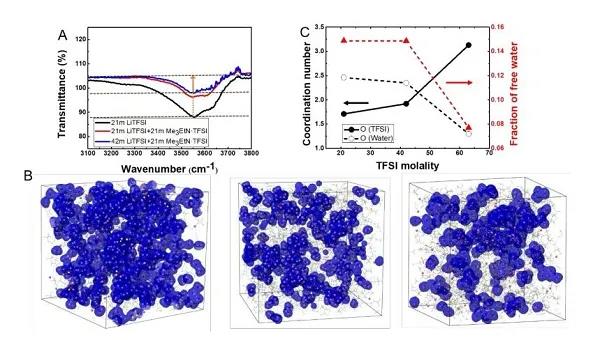
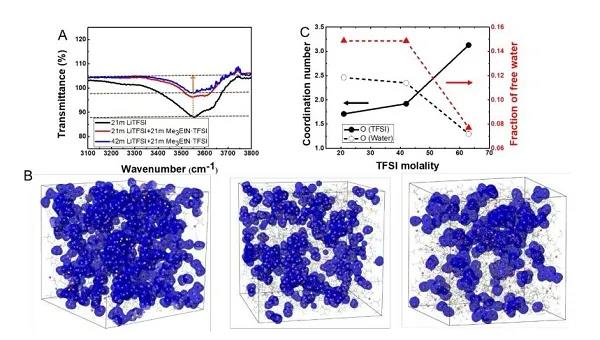
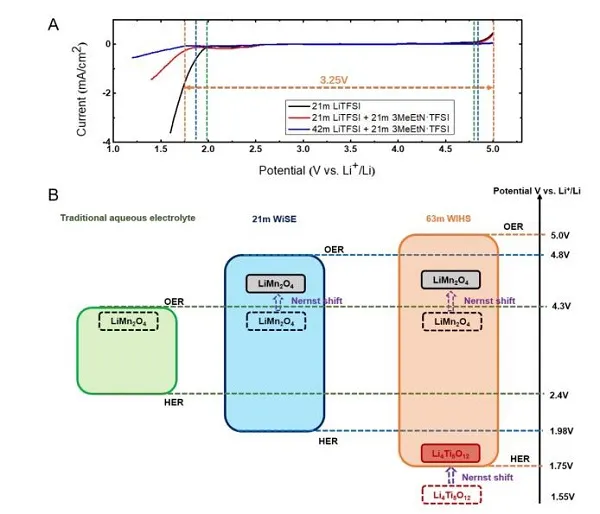
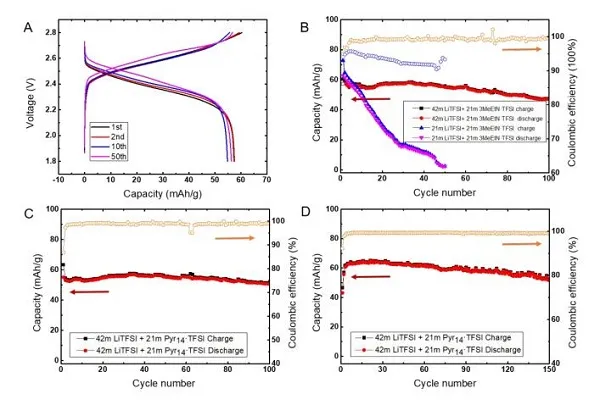
Recently, Dr. Chen Long and others from Professor Wang Chunsheng‘s group at the University of Maryland and others used ethyl trimethylammonium salt (Me3EtN + · TFSI) to increase the solubility of LiTFSI in water by two times. The new water-in-hybrid-salt (WIHS) aqueous electrolyte salt concentration reached an unprecedented 63 m, and the salt / water molar ratio reached 1.13 for the first time. The anomalous phenomenon is that the ammonium salt alone cannot be dissolved in water, but it can be dissolved in an aqueous solution with LiTFSI and can double the solubility of LiTFSI. Despite the high salt concentration, the 63 m WIHS aqueous electrolyte maintains a relatively high ionic conductivity (0.91 mS cm-1) and a relatively low viscosity (407 mPa s), while having a wider 3.25 V electrochemical Stability window. It can be seen from Figure 1C that in the WIHS aqueous electrolyte, the number of water molecules in the Li + solvated structure and in the bulk are greatly reduced. The TFSI- anion ratio in the Li + solvated structure increases, which is more conducive to the growth of SEI. The electrolyte can support a 2.5 V water-based lithium-ion (LiMn2O4 // Li4Ti5O12) full cell with stable cycling at a rate of 1 C and 0.2 C, and the battery energy density can reach 145Wh kg-1. The above results were published in the international top energy journal ACS Energy Letter.
[Picture and text guide]
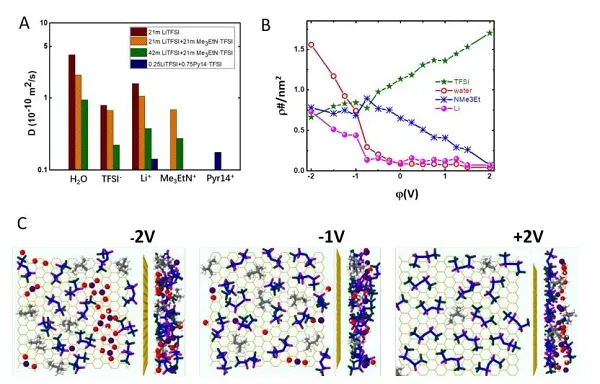
Figure 1. FTIR diagram and molecular dynamics simulation of liquid structure

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |