 【Research Background】
【Research Background】
Potassium ion batteries (PIBs) have a "rocking chair" mechanism similar to lithium / sodium ion batteries (LIBs / SIBs). Because of their low cost, large ionic conductivity in the electrolyte, and high operating voltage, they have been used in large-scale energy storage. extensive attention. Among the anode materials of potassium ion batteries, carbon-based materials have attracted considerable interest from researchers due to their richness, low potential, and low cost, which is crucial for the practical application of PIBs. However, compared with Li +, the kinetics of K + in the host material is slow, which is accompanied by problems such as low initial Coulomb efficiency and poor rate performance in practical applications. From this perspective, N-rich porous carbons (NPCNs) with increased interlayer distances can reduce the barrier to K + intercalation, thereby improving electrochemical performance, and are expected to solve the above problems. In addition, MXene has higher metal conductivity, lower potassium ion diffusion barrier, and negligible volume change during ion intercalation and extraction. It is an electrode material with great research value and potential. Although MXene material has excellent cycle stability and rate performance, for PIBs, the theoretical specific capacity of Ti3C2 MXene is only 191.8mAh / g. At present, Ti3C2 is prone to irreversible agglomeration and stacking, resulting in its actual specific capacity being much lower. Due to theoretical values. In order to solve the above problems, the interlayer structure is optimized, a 2D hierarchical structure is constructed, the self-stacking effect is reduced, additional potassium storage sites are constructed, and a K + fast transmission channel is ensured to effectively improve its reversible capacity and rate performance.
[Achievement Profile]
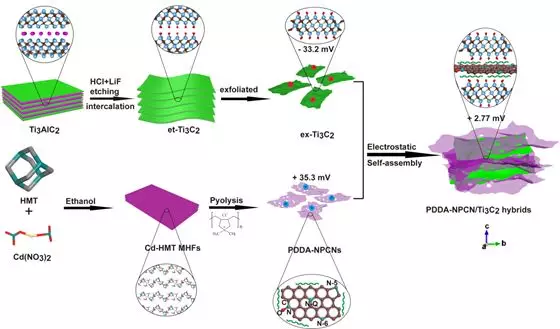
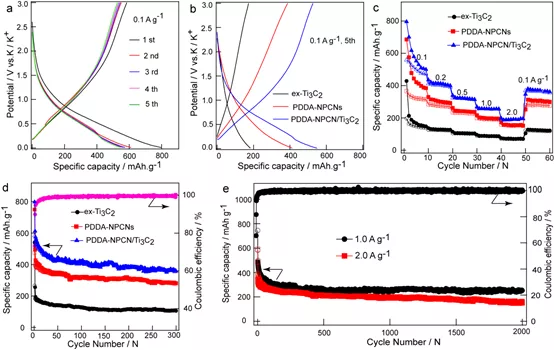
Recently, Professor Yonglong Yin of Shandong University and associate researcher Wang Chengxiang (corresponding author) published a paper titled Self-AssembledTi3C2 MXene and N-Rich Porous Carbon hybrids as Superior Anodes for High-Performance Potassium-Ion in the internationally renowned academic journal Energy & Environmental Science Dr. Ruizhao Zhao, the Batteries research thesis, is the first author of this article. In this study, PDDA-NPCN / Ti3C2 heterojunctions were prepared in a face-to-face manner at the molecular scale by electrostatically assembling a few layers of Ti3C2 MXene and N-rich porous carbon nanosheets (NPCNs). This heterojunction has a laminated structure and a large specific surface area, which can ensure close contact between Ti3C2 and NPCNs, and effectively utilize the two components and more accessible active sites. This heterojunction material provides greater interlayer spacing and a unique three-dimensional interconnected conductive network to accelerate ion / electron transport. This heterojunction material has a significant synergistic effect. After cycling for 300 weeks at 0.1 A g-1, its reversible capacity is 358.4 mA h g-1, and for 2000 weeks at 1.0 A g-1, the attenuation rate is only 0.03%, showing super long cycle stability. DFT calculations further show that the PDDA-NPCN / Ti3C2 complex improves the adsorption capacity of the interface K + and promotes the potassium process.
[Picture and text guide]
Figure 1. Schematic of the synthesis process of PDDA-NPCN / Ti3C2 heterojunction
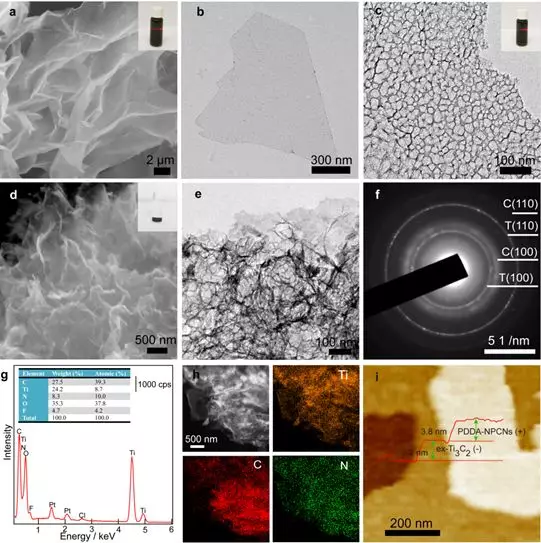
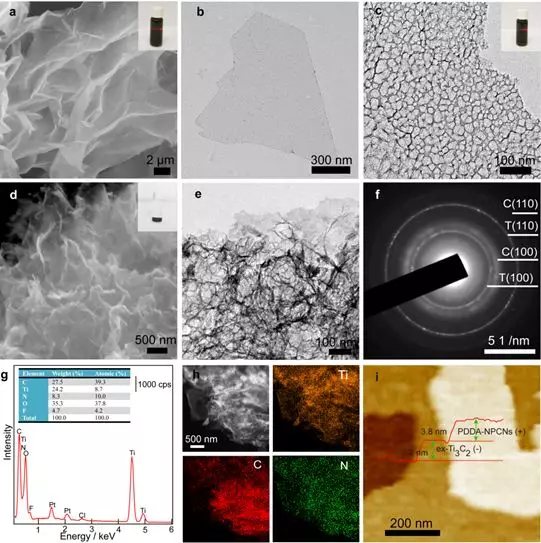
Figure 2. Morphology and chemical characterization. (a-b) SEM and TEM images of ex-Ti3C2 nanosheets. (c) TEM image of PDDA-NPCNs nanosheets. The illustration shows the Tyndall effect. (D) SEM, (e) TEM and (f) ED images of flocculated PDDA-NPCN / Ti3C2. The inset shows a flocculated sample. T stands for former Ti3C2, and C stands for PDDA-NPCNs. (g) EDS energy spectrum. (h) HAADF-STEM image of PDDA-NPCN / Ti3C2 and corresponding element mapping images of Ti, C and N elements. (i) AFM image and thickness profile of PDDA-NPCN / Ti3C2.
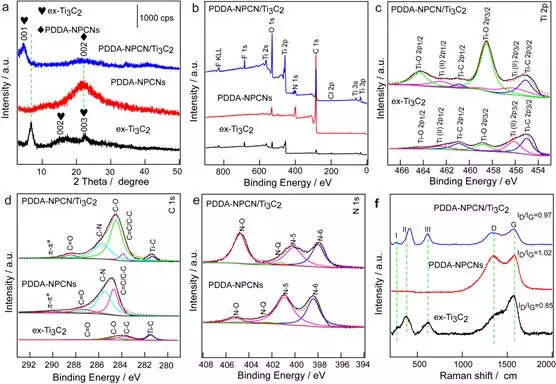
Figure 3. Structural and chemical characterization. (a) XRD patterns of ex-Ti3C2, PDDA-NPCNs and PDDA-NPCN / Ti3C2 heterojunctions. (b-e) XPS spectra of ex-Ti3C2, PDDA-NPCNs and PDDA-NPCN / Ti3C2 heterojunctions. (f) Raman spectra of ex-Ti3C2, PDDA-NPCNs and PDDA-NPCN / Ti3C2 heterojunctions.
Figure 4. Potassium ion storage performance. (a) Constant current charge / discharge curve for the first 5 turns of PDDA-NPCN / Ti3C2 negative electrode at 0.1 A g-1. (b) the fifth charge / discharge curve at 0.1 A g-1, (c) the rate performance of different anodes at different current densities, and (d) the cycle stability curve at 0.1 A g-1. (e) Long-cycle stability curve of PDDA-NPCN / Ti3C2 anode at current densities of 1.0 and 2.0 A g-1.
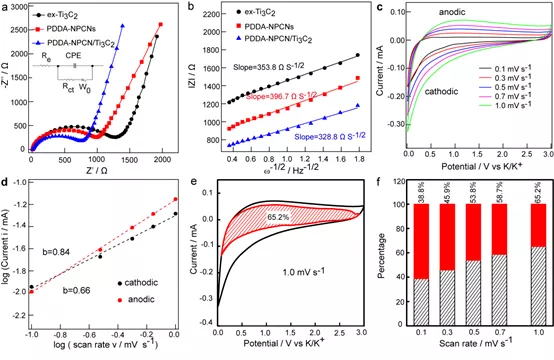
Figure 5. Electrochemical kinetic behavior. (a) Nyquist diagram, equivalent circuit diagram. (b) Figures | Z | vs.ω1 / 2 of different negative electrodes in the Warburg region, respectively. (c) CV curves of PDDA-NPCN / Ti3C2 anode at different scan rates. (d) The relationship between log (i) vs log (v) of PDDA-NPCN / Ti3C2 anode. (e) Capacitance control and diffusion control contribution of PDDA-NPCN / Ti3C2 negative electrode at 1.0mV s-1. (f) The normalized contribution percentage of the capacitance control capacity of the PDDA-NPCN / Ti3C2 negative electrode at different scan rates.
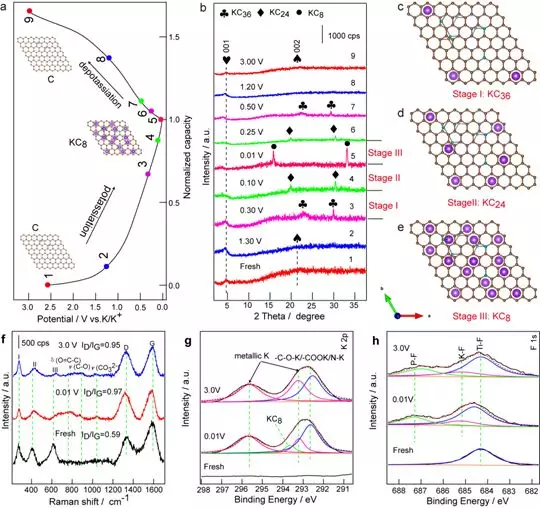
Figure 6 Electrochemical mechanism. (a) The voltage curves of the PDDA-NPCN / Ti3C2 negative electrode in different states and (b) the corresponding quasi-in situ XRD patterns during the initial potassiumation / depotassiumization process. (c) KC36, (d) KC24 and (e) KC8 supercells. (f) Quasi-in-situ Raman spectra and (g-h) quasi-in-situ XPS spectra of PDDA-NPCN / Ti3C2 anodes under different potassium / depotassium states.
[Summary of this article]
In summary, the authors used a simple liquid flocculation and electrostatic adsorption self-assembly method to rationally design a new PDDA-NPCN / Ti3C2 heterojunction material for high-performance potassium ion batteries. This material has excellent potassium storage properties. It has a high reversible capacity of 358.4 mAh g-1 after 300 cycles of 0.1 A g-1, which is much larger than ex-Ti3C2 (106.2 mAh g-1) and NPCNs (280.9 mAh). g-1). This is due to the stacked structure of the heterojunction material, which ensures the close contact between Ti3C2 and NPCNs, so as to effectively play the role of these two parts. PDDA-NPCN / Ti3C2 heterojunction materials provide greater interlayer spacing, a unique 3D porous interconnect conductive network, and full use of active sites, which shortens the pathway for electrolyte ions and promotes rapid ion / Electronic transportation is conducive to improving the performance of materials. The robust heterostructure can alleviate the severe volume change caused by phase change during the rapid charge and discharge process, which helps to maintain the rate performance and cycle stability. DFT calculations further revealed that PDDA-NPCN / Ti3C2 improved the electrochemical performance synergistically by enhancing the interface K + adsorption reaction, and was beneficial to the process of potassium insertion. This method provides a new idea for the controlled exploration of the self-assembly of the coupling compound in an energy storage device.
Literature link:
https://pubs.rsc.org/en/content/articlelanding/2019/ee/c9ee03250a#!divAbstract


 【Research Background】
【Research Background】