|
(Nanowerk Spotlight) Graphene and its derivatives have seen a recent boom in research interest due to the discovery of some quite remarkable properties that occur when chunks of graphite are reduced to a single, two-dimensional (2D) layer of carbon atoms (graphene). These properties include high surface area, excellent conductivity and mechanical strength. Various nanoparticles have been attached to graphene in an attempt to create advancements in a number of fields from sensors to fuel cells (see for instance: "Photoactive graphene-semiconductor composites").
|
|
Carbon-supported catalysts are widely used in many applications. For example, platinum nanoparticles supported on bulk carbon frameworks are used as fuel cell electrodes (see for instance: "Nanotechnology catalysts offer prospect of commercially viable hydrogen fuel cells"; the latest advances use carbon nanotubes for this; see "Carbon nanotube-DNA nanotechnology for improved fuel cell catalysts"). The obvious challenge is to have a large area of carbon surface so that the catalyst particles can be dispersed without any aggregation. Graphene with its 2D nanostructure provides a large surface area (theoretically, the surface area of graphene is about 2600 square meters per gram) to anchor catalyst particles.
|
|
Previous work has shown that suspension-based sheets of functionalized graphene – or graphene oxide – provides a convenient way to to keep sheets exfoliated and available for ion or nanoparticle intercalation. And although single nanoparticle systems like gold-on-graphene or platinum-on-graphene have been achieved using graphene oxide substrates, researchers found that, compared to pure graphene, graphene oxide suffers from a significant loss of conductivity. But they also found that a partial reduction of its functional groups mitigates this problem. Nevertheless, so far only few studies have examined using reduced graphene oxide (RGO) as a substrate for catalytic systems.
|
|
In new work that was funded by the Office of Basic Energy Sciences, US Department of Energy, scientists at the University of Notre Dame have now succeeded in dispersing two different types of nanoparticles – silver and titanium dioxide – on a reduced graphene oxide at different sites without any aggregation.
|
|
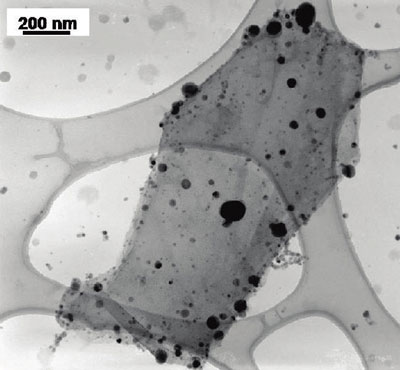
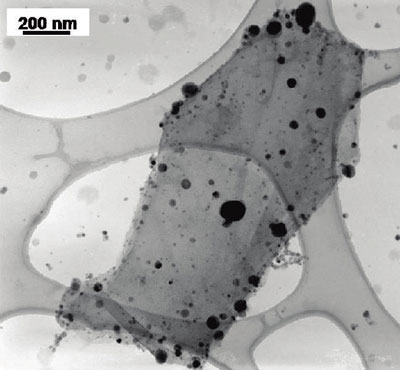
"Using reduced graphene oxide as a two-dimensional support we have succeeded in selective anchoring of semiconductor and metal nanoparticles," Prashant V. Kamat tells Nanowerk. "The photogenerated electrons in titanium dioxide are transferred to graphene and these electrons are transported across graphene sheet to reduce silver ions. The growth of silver nanoparticles confirm the ability of graphene sheet as a electronic communicating platform between semiconductor and metal nanoparticles anchored on a graphene sheet."
|
|
|
TEM image of titanium dioxide (smaller size) and silver (larger and darker) particles deposited on a single reduced graphene oxide sheet (Image: Dr. Kamat, University of Notre Dame)
|
|
With this novel work, Kamat, a professor of chemistry & biochemistry, together with his team, has demonstrated a relatively simple, light-driven and solution-processable process to form graphene systems with both semiconductor and metal nanoparticles anchored at separate sites on the graphene sheet. The incorporation of both types of nanoparticles provides a greater flexibility of function.
|
|
The work also demonstrates graphene‘s ability to store and shuttle electrons – the graphene sheet facilitates direct communication between different nanoparticles by shuttling electrons across the carbon plane. Kamat points out that it shouldn‘t be difficult to deposit other catalyst nanoparticles in order to incorporate additional selectivity.
|
|
The findings indicate that graphene has the potential to become the ideal substrate for a number of catalytic and sensing processes.
|
|
The researchers have reported their findings in the January 7, 2010 online issue of Nano Letters("Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Catalyst Mat. Storing and Shuttling Electrons with Reduced Graphene Oxide").
|
|
"Incorporation of two or more catalyst particles onto an individual graphene or reduced graphene oxide sheet at separate sites provides greater versatility in carrying out selective catalytic or sensing processes," explains Kamat. "One such process we illustrate is water splitting catalyst system where oxygen is generated at photocatalyst (Cat1) and hydrogen is generated at metal catalyst (Cat 2). Another area is in the development of next generation sensors with greater sensitivity and selectivity."
|
|
Kamat notes that the development of a graphene-based multifunctional catalyst mat is a relatively new concept but one that offers unique opportunities in developing sensor and catalyst systems: "Our current focus is to utilize them in a water-splitting catalyst assembly in which reduction and oxidation can be carried out at two separate sites. In addition we are exploring stacked graphene/metal oxide sheets to develop new electrode materials for solar cells and batteries. This maiden study can encourage future ‘graphitic designers‘ to develop new tailored platforms in nanotechnology."
|