【introduction】
With the rapid development of modern society and economy, the global demand for energy is increasing exponentially over the past few decades, causing people to pay close attention to the energy crisis. Among various energy storage technologies, lithium-ion batteries (LIBs) have attracted wide attention due to their advantages such as high energy density, low cost, and environmental friendliness. However, due to the high reactivity of lithium metal, the widespread application of lithium metal-based LIBs still faces great challenges, such as low coulomb efficiency, short cycle life, and safety issues caused by lithium dendrites. During the charge and discharge process, the uniform deposition of lithium ions on lithium metal is the key to suppress the formation of lithium dendrites. Studies have shown that solid electrolytes based on single ion conductors are one of the effective methods to solve the lithium dendrite problem, except for the typical lithium phosphorus oxynitride (LiPON) and Li 4-x Ge 1-x P x S 4 (thio-LISICON In addition, porous coordination polymer metal organic frameworks (MOFs) have also attracted much attention as single ion conductors. Compared with other traditional porous carbon-based materials and inorganic oxide materials, MOF not only has rich cavity structure and high specific surface, but also has many inherent advantages, such as its highly ordered porous structure, controlled pore size and topological structure, and Inorganic-organic hybrid properties. Due to these advantages, MOF is able to store high-density charges in a smaller volume. This is beneficial to achieve dense cation jump sites, to minimize the activation energy of ion transport, and thereby improve ion conductivity. In addition, after removing the solvent molecules of the MOF metal site, a large number of unsaturated cation coordination sites will be exposed, attracting anions to bind to them, and achieving high metal ion conductivity. Due to its rich cavity structure, MOF can also serve as a host to accommodate a variety of liquid / gaseous materials. Therefore, MOFs with appropriate pore sizes can be introduced into batteries as ion sieves to regulate ion transmission. In addition, the uniform and ordered microporous structure of MOF helps to regulate the uniform deposition of lithium ions, thereby inhibiting the formation of lithium dendrites.
[Achievement Profile]
Recently, the research group of Zhou Haoshen of Nanjing University and a foreign research team have jointly analyzed and discussed the effect of MOF-based diaphragms on ion transmission. Simulation calculations and experimental results show that liquid electrolyte filled MOF films can help guide the uniform deposition of lithium ions, thereby Inhibits the growth of lithium dendrites. At the same time, the research group also summarized the latest research results of MOF films in lithium-metal batteries and lithium-ion battery systems. When the MOF-based separator is used in Li-S batteries, it can effectively inhibit the charge-discharge intermediate product, polysulfide ion, to extend the cycle life of the battery; when the MOF-based separator is used in Li-O 2 batteries, it can It is used to develop the double redox medium strategy to improve the electrochemical performance of the battery. When MOF-based separators are used in lithium-ion batteries, it helps to develop the battery and electrolyte system to achieve high-voltage battery systems. In addition, based on the research progress made in rechargeable lithium batteries, the authors propose the application prospects of MOF-based separators in sodium-metal batteries, organic redox flow batteries, and liquid negative batteries. At the end of the review, the author provides suggestions for designing and preparing high-quality MOF films and using them in rechargeable battery systems from a practical perspective. Related research results were published on Energy Environ. Sci. Under the title " The Potential of Electrolyte Filled MOF Membrane as Ionic Sieve in Rechargeable Batteries " .
[Picture and text guide]
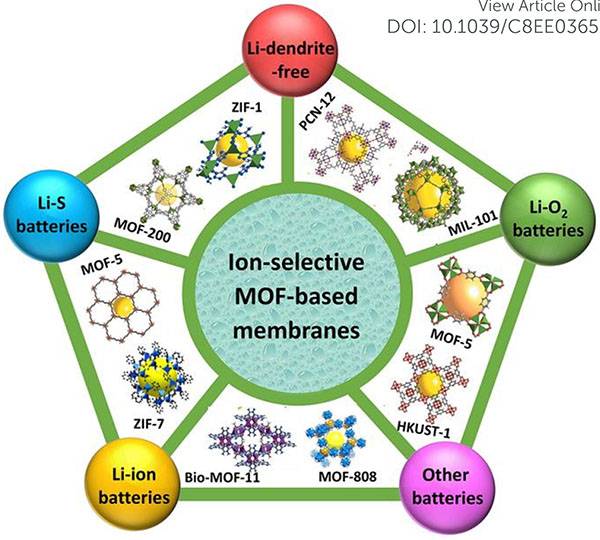
Figure 1. M OF membrane with ion selectivity applied to various battery systems, and some representative M OF structure diagrams
1. Preparation of MOF membrane
The high quality and defect-free MOF membrane is the key to its application as an ion sieve in battery systems. In the past few decades, researchers have made great efforts in preparing high-quality MOF films. According to the type of the prepared film, the technology for preparing the MOF film is summarized into three parts: a self-supporting MOF film, a MOF coating-base film, and a MOF-based composite film.
1.1 Preparation of self-supporting MOF film by self-assembly method
Figure 2. Preparation of MOF film by interface assembly method (a) Synthesis of MOF layer at a biphasic interface containing an aqueous solution of metal ions and an organic ligand solution;
(a) Synthesis of MOF layer at a biphasic interface containing an aqueous solution of metal ions and an organic ligand solution;
(B) The interface diagram of the synthesized MOF layer;
(C) Schematic of NAFS-13 nanosheets prepared by delayed injection.
1.2 Deposition of MOF coating on substrate
Figure 3.Introduction of M OF coating on substrate
(A) A schematic diagram of the general strategy for preparing MOF by vapor deposition;
(B) A schematic diagram of introducing a Cu 2 (BDC) 2 MOFs layer on a Cu (OH) 2 substrate using a heteroepitaxial growth method ;
(C) A schematic diagram of a spin coating device;
(D) Schematic diagram of the preparation of MOF EPD film by external electric field;
(E) HKUST-1 has different growth direction selectivity on SAM-modified gold surface substrates modified with OH- and COOH-.
1.3 Construction of MOF-based composite membrane
Figure 4. Preparation of MOF-based composite film
(A) Schematic diagram of the process of preparing MOF @ GO membrane by vacuum filtration;
(B) Digital photo of flexible MOF @ PVDF-HFP diaphragm;
(C) A schematic diagram of the process of preparing a MOF-based composite film by a doctor blade flow method;
(D) Digital photo of MOF-based composite film prepared by doctor blade extension method.
2. Stable lithium deposition
In lithium batteries using lithium metal as a negative electrode, lithium dendrite growth is caused by heterogeneous deposition of lithium ions. The growth of lithium dendrites increases the reaction area of lithium metal and electrolyte side reactions, accelerates the consumption of active lithium and electrolyte, causes rapid decline in battery capacity, and shortens battery life. What‘s more serious is that the sharp lithium dendrite can pierce the battery separator, cause a short circuit inside the battery, and bring serious safety risks.
Figure 5. MOF-based separator / electrolyte system effectively regulates the homogeneous deposition of lithium ions (a) Schematic diagram of lithium metal dendrite growth caused by heterogeneous transport of lithium ions (above), MOF-based separator / electrolyte achieves uniform Lithium ion transport and homogeneous lithium deposition to obtain stable and efficient lithium metal electrodes (below);
(a) Schematic diagram of lithium metal dendrite growth caused by heterogeneous transport of lithium ions (above), MOF-based separator / electrolyte achieves uniform Lithium ion transport and homogeneous lithium deposition to obtain stable and efficient lithium metal electrodes (below);
(B) TFSI - a schematic diagram of passing through the MOF channel along two different paths;
(C) Calculate the energy barrier for TFSI - anions to migrate along the two pathways in (b);
(D) Changes of MSD of Li + and TFSI‒ ions in MOF-based electrolytes over simulation time;
(E) Schematic diagram of the ion sieves of MOF-based electrolytes.
Figure 6. Performance test of Li // Li symmetrical battery (a)Comparison of lithium deposition / peeling behavior in original electrolyte and MOF-based electrolyte under the test conditions of10 mA cm -2 and 10 mA h cm -2 ;
(a)Comparison of lithium deposition / peeling behavior in original electrolyte and MOF-based electrolyte under the test conditions of10 mA cm -2 and 10 mA h cm -2 ;
(B) SEM images after the lithium metal cycle using a PP separator and a MOF-based separator, respectively;
(C) Li // Li symmetrical battery with MOF-based separator when the current density is increased from 0.5 to 20 mA cm -2 ;
(D) Comparison of cycling stability of Li // Li symmetrical batteries assembled with different separators at high temperatures.
Figure 7. Electrochemical performance of Li // Cu batteries (a) Coulomb efficiency of continuous deposition / stripping cycles under different current densities;
(a) Coulomb efficiency of continuous deposition / stripping cycles under different current densities;
(B) Coulomb efficiency of Li deposition / stripping under the conditions of 0.25 mA cm -2 and 1.0 mA h cm -2 ;
(C) Measurement of steady state current and lithium ion migration number in 1.0 M LiTFSI in DOL / DME with 2% LiNO 3 electrolyte filled NH 2 -MIL-125 (Ti) diaphragm system.
3. Application in lithium metal batteries
In addition to inhibiting lithium dendrite growth, in some new rechargeable lithium metal batteries, such as lithium metal batteries, lithium sulfur batteries, and lithium oxygen batteries assembled from intercalated cathode materials, the ion-selective MOF-based film can also suppress other Adverse side effects.
3.1 Lithium metal battery with intercalation cathode material
Figure 8. Cycle performance of Li-Li 4 Ti 5 O 12 battery (a)Cycle stabilityof Li-Li 4 Ti 5 O 12 batteryin original electrolyte and MOF @ GO electrolyte;
(a)Cycle stabilityof Li-Li 4 Ti 5 O 12 batteryin original electrolyte and MOF @ GO electrolyte;
(B) The charge / discharge curve of the battery using MOF @ GO-based electrolyte.
3.2 Lithium-sulfur battery
Figure IX. Schematic diagram of different separators used in Li-S batteries. (A) A series of problems caused by conventional separators cannot suppress the shuttle effect of polysulfides and the growth of lithium dendrites in lithium-sulfur batteries.
(A) A series of problems caused by conventional separators cannot suppress the shuttle effect of polysulfides and the growth of lithium dendrites in lithium-sulfur batteries.
(B) The MOF @ PVDF-HFP separator with a highly ordered porous structure can not only block the diffusion of polysulfide ions, but also guide the uniform deposition of lithium ions, thereby inhibiting the growth of lithium dendrites.
Figure 10. Cycle performance of Li-S batteries (a) Optical image of a visual H-type Li-S battery assembled with a MOF @ PVDF-HFP diaphragm during discharge;
(a) Optical image of a visual H-type Li-S battery assembled with a MOF @ PVDF-HFP diaphragm during discharge;
(B) Cycle performance of Li-S batteries with different separators;
(C) Cycle performance of Li-S batteries using MOF @ PVDF-HFP / GO separators under different sulfur loadings;
(D) Cycle performance of flexible Li-S soft pack batteries assembled using MOF @ PVDF-HFP separators;
(E) FT-IR spectra of MOF @ GO diaphragm before and after cycling.
3.3 Lithium-oxygen battery
Figure 11: Performance of Li-O 2 assembled based on MOF separator (a) Schematic diagram of developing double redox media strategy using MOF-based separator;
(B) The voltage distribution of Li-O 2 batteries under different conditions. The cut-off capacity is set to 0 mAh, the cut-off voltage is in the range of 2.0-4.5 V, and the current density is 1000 mA g -1 ;
(C) In-situ Raman spectra of positive electrodes in different lithium-oxygen batteries;
(D) Performance of a Li-O 2 soft-pack battery assembled using a MOF-based separator at a current rate of 2 mA (cut-off capacity 20 mA h).
4. Application in Li-ion battery
Figure 12. Composite electrolyte system (a) Schematic diagram of a composite electrolyte system incorporating a MOF-based separator;
(a) Schematic diagram of a composite electrolyte system incorporating a MOF-based separator;
(B) Penetration test using MOF-based separators to evaluate the barrier effect of the separators on dual electrolytes;
(C) The charge and discharge curve of the combined electrolyte;
(D) Long-cycle performance of introducing MOF-based separator / combined electrolyte in full cells.
5. Potential among other rechargeable batteries
5.1 Sodium ion battery
Figure 13. Schematic diagram of sodium deposition / stripping (a) Schematic diagram of sodium metal failure during repeated deposition / stripping;
(a) Schematic diagram of sodium metal failure during repeated deposition / stripping;
(B) The introduction of MOF-based separators with uniform pore diameters can achieve a uniform Na + flux, fundamentally solve the problem of Na dendrite, and achieve stable Na ion deposition.
5.2 Organic Redox Flow Battery
Figure 14.Simplified schematic diagram of an organic redox flow battery using an ion-selective MOF-based separator
5.3 Liquid negative battery
Figure 15.Simplified schematic diagram of a liquid-negative battery using an ion-selective MOF-based separator
【summary】
In summary, this article summarizes various advanced methods for building high-quality MOF films, including self-supporting MOF films, MOF coatings / base films, and MOF-based composite films. At the same time, the latest research progress of MOF-based films in lithium metal batteries and lithium-ion battery systems is summarized. Although significant progress has been made in the construction of MOF-based films, the technology for making high-quality MOF films still needs to be further improved. From the perspective of practical application, this article makes several suggestions:
(1) So far, more than 20,000 MOFs have been successfully prepared by designing different inorganic metal ion / cluster and organic connection methods, but only a few MOFs have been used to prepare battery separators. This is due to some metals in MOF Ions may react with active materials in the battery, resulting in rapid decay of battery capacity. Based on this, the compatibility of MOF with various batteries should be carefully examined before designing the MOF film, and proper pretreatment can effectively avoid adverse reactions and ensure battery performance;
(2) In order to achieve the high energy density of rechargeable lithium batteries, the MOF-based film introduced should be as light as possible to reduce the impact of inactive materials on the energy density of the battery;
(3) The measurement of ionic conductivity is an important factor. The membrane‘s proper porosity and good electrolyte wettability are beneficial for improving electrolyte absorption and storage. In addition, the thickness of the separator also greatly affects its ionic conductivity. The addition of suitable additives can also help to form a MOF film with high ionic conductivity;
(4) Although the current technology can obtain high crystallinity, close packing and uniform distribution of MOF crystals, its mechanical properties are still difficult to meet the harsh working environment. In addition, complex synthetic processes can be easily implemented in the laboratory, but large-scale production in the factory still faces challenges;
(5) The mechanism and electrochemical interaction of MOF as an electrolyte or ion sieve in a rechargeable battery should be better understood.
In general, although the potential of MOF membranes still needs to be explored and tapped, it is expected to be applied to advanced storage systems through further development and innovation in the future.
【team introduction】
Zhou Haoshen: Professor of Nanjing University, specially invited expert of National "Thousand Talents Plan", Changjiang Scholar, 973 chief scientist, concurrently chief researcher of Japan National Institute of Industrial Technology (AIST) and professor of Tsukuba University. Engaged in research and development of lithium ion batteries, sodium ion batteries, lithium air batteries, lithium sulfur batteries, lithium flow batteries and solid state batteries. In Nat. Mater .; Nat. Energy, Nat. Commun. (5 articles); Joule (5 articles); Angew. Chem. Int. Ed. (13 articles); Energy Environ. Sci. (26 articles); J. Am. Chem. Soc. (5 papers); Adv. Mater. (16 papers); Adv. Energy Mater. (18 papers) and more than 400 papers published in top international academic journals. He cited more than 33350 times, H factor 97.

He Yibai: He graduated from Shaanxi Normal University in July 2016 with a master‘s degree in engineering; in October of the same year, he entered the research group of Professor Zhou Haoshen of the University of Tsukuba in Japan for a doctorate. The main research area is the development of functional separators for various lithium-metal batteries based on metal lithium anodes. The first author has published many articles in international high-level journals in the field of energy materials and electrochemistry such as Energy Environment Sci. And Adv. Energy Mater.
[ Summary of team work in this field ]
-
Bai, X. Liu, K. Zhu, S. Wu and H. Zhou *, Metal–organic framework-based separator for lithium–sulfur batteries. Nature Energy, 1, 16094.
-
Bai, Y. Sun, J. Yi, Y. He, Y. Qiao H. Zhou *, High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2018, 2, 2117.
-
He, Y. Qiao, Z. Chang and H. Zhou *,The Potential of Electrolyte Filled MOF Membrane as Ionic Sieve in Rechargeable Batteries. Energy & Environmental Science, 2019. DOI: 10.1039 / C8EE03651A
-
He, Z. Chang, S. Wu, Y. Qiao, S. Bai, K. Jiang, P. He and H. Zhou *,Simultaneously inhibiting lithium dendrites growth and polysulfides shuttle by a flexible MOF-based membrane in Li-S batteries. Advanced Energy Materials , 2018, 1802130.
-
Qiao, † Y. He, † K. Jiang, Y. Liu, X. Li, M. Jia, S. Guo and H. Zhou *, High-voltage Li-ion full-cells with ultralong term cycle life at elevated temperature . Advanced Energy Materials , 2018, 1,802,322.
-
Qiao, † Y. He, † S. Wu, K. Jiang, X. Li, S. Guo, P. He and H. Zhou *, MOF-based separator in an Li-O 2 battery: an effective strategy to restrain the shuttling of dual redox mediators. ACS Energy Letters , 2018, 3, 463.
-
Bai, K. Zhu, S. Wu, Y. Wang, J. Yi, M. Ishida and H. Zhou *, A long-life lithium--sulphur battery by integrating zinc--organic framework based separator. Journal of Materials Chemistry A, 2016, 4, 16812.
Literature link: " The Potential of Electrolyte Filled MOF Membrane as Ionic Sieve in Rechargeable Batteries " (Energy Environ. Sci. , 2019, DOI: 10.1039 / C8EE03651A.)
Source of information: material cattle





















