¡¾Research Background¡¿
In the 21st century, it is difficult for mankind to imagine a world without various wearable electronic devices. Considering the increasing energy consumption demand for such smart devices and the growing shortage of fossil energy, clean energy systems that can be stored in large quantities are very important. Although the secondary battery has considerable energy density, its limited power density limits its application in some specific fields. At the same time, the ultra-high power density and cycle stability of supercapacitors cannot offset the shortage of energy density. At present, researchers all over the world are committed to exploring new materials with high energy density and high power density. Among them, two-dimensional (2D) materials have high thickness-to-diameter ratios due to their outstanding electronic, mechanical and optical properties. Its atomic thickness makes 2D materials have broad prospects in many applications. In addition to single atom 2D materials, such as graphene, silicon, germane, and phosphorene, most include two or more elements, such as transition metal chalcogenides (TMDs) and hydrotalcite bimetallic hydroxides ( LDHs), especially the new graphene-like 2D materials first etched by Professor Yury Gogotsi¡®s research group in 2011, has developed rapidly in the past decade, and is a hot star in the 2D material family. Recently, NR Hemanth of Indian Institute of Technology and Professor Balasubramanian Kandasubramanian of Department of Metallurgy and Material Engineering of National Defense High-Tech Research Institute published a review article in the internationally renowned academic journal Chemical Engineering Journal , entitled: Recent advances in 2D MXenes for enhanced cation intercalation in energy harvesting Applications: A review . This review system summarizes the synthesis, structure, intercalation, layering, properties of MXenes, and the relationship between nanostructures and electrochemical performance, which provides a reference for the future research direction of 2D MXenes.
¡¾Graphic introduction¡¿

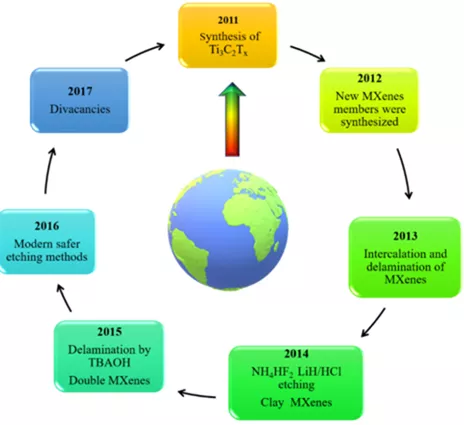
Figure 1. MXenes material development timeline over the past decade

Figure 2. The crystal structure of different types of MXenes materials, the surface functional group is hydroxyl (-OH)

Table 1. Synthesis conditions and lattice parameters of different MAX phases and MXenes

Figure 3. Side view of the structure of the MXenes material, the SEM image of the MXenes obtained by HF etching and the in-situ synthesis HF etching method.

Figure 4. a) A few layers of Ti 3 C 2 paper obtained by suction filtration ; b) XRD pattern before and after HF etching; c) MXenes film prepared by spray coating on a highly transparent substrate; d) -e) Single-layer and double-layer Ti 3 C 2 T x .

Figure 5. 6-coordinate ion diamond lattice of Ti 3 C 2 layer.

Figure 6. The band gap at various Fermi levels of MXenes at zero.

Figure 7. Layered Ti 3 C 2 with oxygen functional groups . b1, b2 Ti 3 C 2 O 2 lithiation process. c1, c2: additional lithium layer on the surface of Ti 3 C 2 O 2 Li 2

Figure 8. a) TEM image of polypyrrole @ Ti 3 C 2 T x composite, b) SEM image of PVP-Sn @ Ti 3 C 2 composite, c) SEM image of CNT-Ti 3 C 2 T xcomposite . e) Cyclic stability test at different current densities and their corresponding Coulomb efficiency.

Table 2. Other applications of MXenes materials besides energy storage.
¡¾Summary and Outlook¡¿
Thanks to the synergistic effect of preventing agglomeration, enhancing electrochemical durability, enhancing pseudocapacitance , and improving electronic conductivity, MXene -based electrodes have opened the door to diverse energy storage systems. The two-dimensional morphology and interface characteristics of Ti 3 C 2 T x can promote the intercalation of many multivalent cations. Li-ion intercalation has received extensive attention in recent years in the application of lithium-ion batteries. In addition to Li ions, MXene is a potential host for intercalation of other cations including Na + , K + , NH 4+ , Mg 2+ and Al 3+ by electrochemical means, which can reach a capacity of more than 300 F The negatively polarized Ti 3 SiC 2 can reach a higher area capacity at different current densities. The combination of MXene and transition metal oxide is also one of the ideal choices for electrode materials. Because of its high capacity, the combination of transition metal oxide, CNT and PPy heterostructures can fully suppress the re-stacking of MXene nanosheets. In addition, the porous structure of MXene can improve the performance of supercapacitors. In addition to symmetrical supercapacitors, other types of supercapacitors should also have good application prospects.
Literature link:
https://doi.org/10.1016/j.cej.2019.123678
Source: MXene Frontier














