
On April 13, 2020, "Nature Materials" published the latest research results of the laboratory of Teacher Huang Qing of Ningbo Institute of Materials Science in the field of MXene material synthesis and energy storage "A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non -aqueous electrolyte "(DOI: 10.1038 / s41563-020-0657-0). The research was carried out by researchers from the laboratory of Teacher Huang Qing of the Ningbo Institute of Materials and the cooperative teams of the University of Toulouse in France, Sichuan University and Linköping University in Sweden.
Two-dimensional (2D) transition metal carbides or carbonitrides (MXenes) are one of the newest members of the 2D material family. They are prepared by selectively etching the A atomic layer in the precursor M n + 1 AX n phase, where M Early transition metal elements (Ti, V, Nb, etc.), A is mainly group 13-16 elements (Al, Si, etc.), X is carbon and / or nitrogen. With its unique 2D layered structure, hydrophilic surface and metal conductivity (> 6000 S cm -1 ), MXenes shows great application prospects in many fields, especially in the field of electrochemical energy storage. After the first synthesis of Ti 3 C 2 MXene in 2011, the preparation of MXenes mainly etched the Al atomic layer of the MAX phase by a solution containing fluoride ion, such as aqueous solution of hydrofluoric acid (HF), a mixture of lithium fluoride and hydrochloric acid (LiF + HCl) or ammonium bifluoride ((NH 4 ) HF 2 ). Therefore, the current challenges of MXene synthesis are as follows: 1) Looking for a green, safe, and fluorine-free synthesis route to prepare MXenes, 2) Exploring MXenes corresponding to the unconventional Al element MAX at the A site, and expanding the range of MAX phase precursors (currently reported There are more than 80 types of MAX phases, and only a few are used to synthesize MXene, of which the most commonly used is Ti 3 AlC 2 ). Therefore, the development of new transition metal carbide precursors and the exploration of new etching methods and mechanisms are of great significance for expanding the surface chemical properties, structural diversity, controllable synthesis and potential applications of two-dimensional transition metal carbides.
Researcher Huang Qing from the laboratory of Teacher Huang Qing of Ningbo Institute of Materials Science and Technology led the scientific research team for years Its applied research. In the early stage, the ternary transition metal carbide Zr 3 Al 3 C 5 precursor was etched by fluorine-containing solution for the first time to successfully prepare the transition metal carbide material Zr 3 C 2 T x with rich d electronic structure ( Angewandte Chemie International Edition, 128 (16 ) , 2016), Hf 3 C 2 T x ( ACS Nano 11 (4), 2017 ) and ScC x OH ( ACS Nano 13 (2), 2019 ). In 2019, laboratory researchers discovered that the Ti 3 AlC 2 MAX phase will undergo a significant reaction in the ZnCl 2 molten salt. The Zn 2+ cation acts as a Lewis acid in the HF acid H +The role of Cl - anion is equivalent to F - eventually coordinated with the M atom. Therefore, the laboratory not only obtained a series of M n + 1 ZnX n phases through this A-site element substitution reaction , but also realized a M n + 1 X n Cl 2 MXene two-dimensional material with a Cl group on the surface ( Journal of the American Chemical Society , 141 (11) , 2019). Can this method be extended to more MAX phase family materials and a wider range of inorganic Lewis acid systems? Can appropriate chemical criteria be established to make Lewis acid molten salt stripping more general? This has become the focus of recent research by researchers in this laboratory.
In this study, laboratory researchers successfully extended this stripping strategy to a variety of Lewis acid chloride molten salts (ZnCl 2 , FeCl 2 , CuCl 2 , AgCl, etc.) and broader members of the MAX phase family (such as element A For Al, Zn, Si, Ga, etc.), by constructing the Gibbs free energy mapping map of the redox potential / displacement reaction of cations and element A under high temperature molten salt environment, a Lewis acid molten salt etching MAX phase synthesis is proposed The general strategy of 2D MXene. In order to verify the guidance of the MXene stripping pattern, the researchers conducted a detailed study using the Ti 3 SiC 2 MAX phase. Ti -Si in Ti 3 SiC 2 has stronger metal bonds than Ti-Al, and is generally not used as a MXene precursor. This study found that 700 O cupric chloride C in the molten salt, a Cu / a Cu 2+ oxidation-reduction potential of -0.43 eV, and the Si / Si 4+ oxidation-reduction potential of -1.38 eV. Therefore, the ionized Cu 2+ in the molten salt can easily oxidize the Si atoms to Si 4+ , and Si 4+ will eventually form SiCl 4 gas with Cl − to escape from the Ti 3 C 2 sublayer, while Cu2+ is reduced to Cu elemental. The residual Cu in the product can be removed by ammonium sulfate solution, etc., and finally Ti 3 C 2 T x (T x = Cl, O) MXene containing Cl and O mixed functional groups on the surface is obtained . The researchers further judged the ability of the A-site element to obtain electrons from the Lewis acid salt based on the electrochemical redox potential of the A-site element in the MAX phase and the Lewis acid salt in the chloride, so that a suitable Lewis acid molten salt and MAX phase system can be selected To prepare MXene. The MAX phases such as Ti 2 AlC, Ti 3 AlC 2 , Ti 3 AlCN, Nb 2 AlC, Ta 2 AlC, Ti 2 ZnC and Ti 3 ZnC 2 can be passed through suitable chloride molten salts (CdCl 2 , FeCl 2 , CoCl 2 , CuCl 2 , AgCl, NiCl 2 ) stripped to get the corresponding (Ti 2 CT x , Ti 3CNT x , Nb 2 CT x , Ta 2 CT x , Ti 2 CT x , Ti 3 C 2 T x ) MXenes. The synthesis of MXene material by the Lewis acid molten salt stripping method is different from the mainstream solution of solution stripping (such as the widely used HF acid), which greatly improves the chemical safety of the experimental process and reduces the difficulty and cost of waste liquid disposal. Research progress in the fields of energy storage, catalytic chemical engineering, communication electromagnetic signal management, and biodiagnosis.
In this work, Prof. Huang Qing‘s research team worked closely with Prof. Simon Patrice of the University of Toulouse in France and Prof. Zifeng Lin of Sichuan University to carry out systematic energy storage electrochemical studies using MXene materials stripped from Lewis acid molten salt. The study found that the electrochemical lithium storage performance of accordion-like Ti 3 C 2 T x MXene in LiPF 6 organic electrolyte obtained by stripping Ti 3 SiC 2 from CuCl 2 molten salt was as high as 738 C at 0.5 mV s -1 g -1 (205 mAh g -1 ), and has a large voltage window of 2.8 V (0.2 to 3 V vs. Li + / Li), which is the best pseudo for Ti 3 C 2 T x MXene in organic electrolyte Capacitor lithium storage performance. The MXene has excellent cycle stability, and the capacity retention rate reaches 90% after 2,400 cycles. The above electrochemical tests show that the MXene electrode material prepared by the Lewis acid molten salt stripping strategy can achieve high capacity, high rate charge and discharge (<1 min) and large potential window (0.2–3V versus Li + / without subsequent single-piece stripping) Li) ‘s excellent performance, which paves the way for the practical application of MXene in electrochemical energy storage systems (batteries and lithium ion capacitors).
The first authors of this work are the laboratory doctoral student Li Youbing (instructor Huang Qing researcher) and the French Toulouse University doctoral student Shao Hui (instructor Professor Patrice Simon). Li Youbing is also the first prize winner of the "Fanghua Youth Science Award" set up by Academician Chai Zhifang in our laboratory. This work was funded by the Ningbo Top Talent Program (Advanced Energy Materials Cross-Innovation Team), the National Natural Science Foundation of China, the PIFI International Cooperation Project of the Chinese Academy of Sciences, and the Zhejiang Double Innovation Team Program.
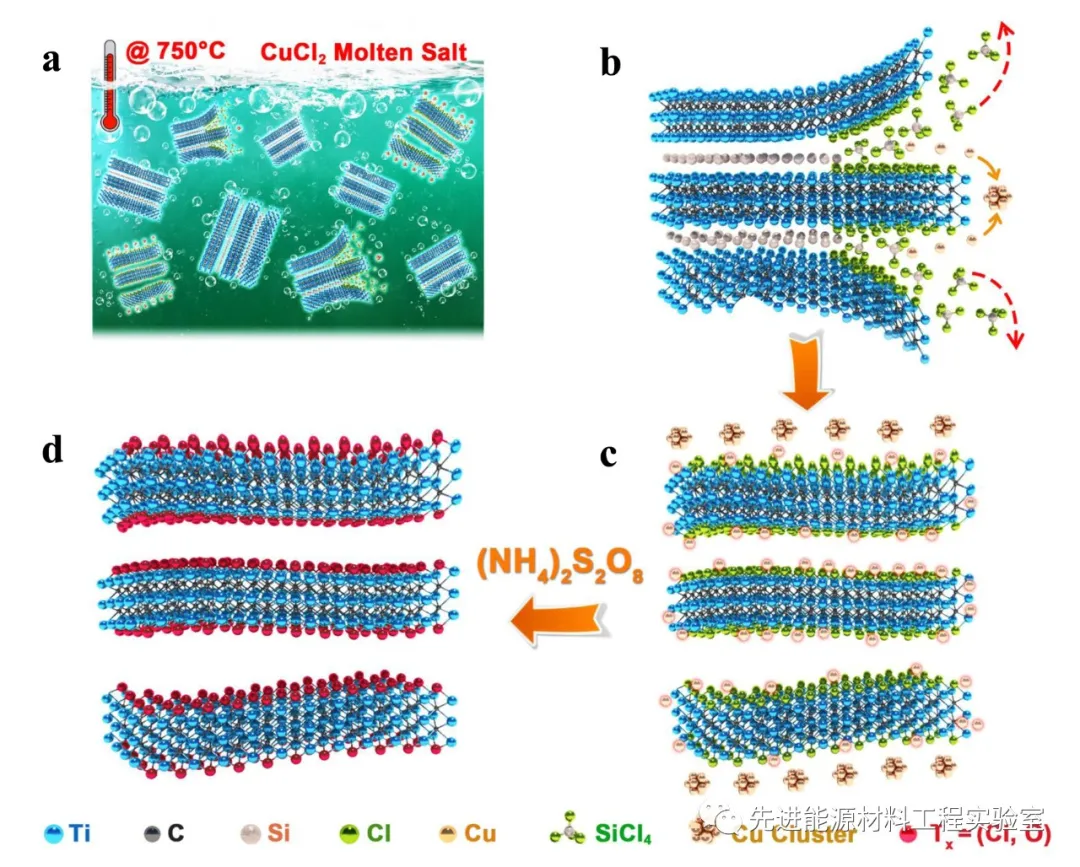
Figure 1 Schematic diagram of synthesis of MXene by Lewis acid molten salt: Taking CuCl 2 etching Ti 3 SiC 2 to synthesize Ti 3 C 2 T x MXene as an example.
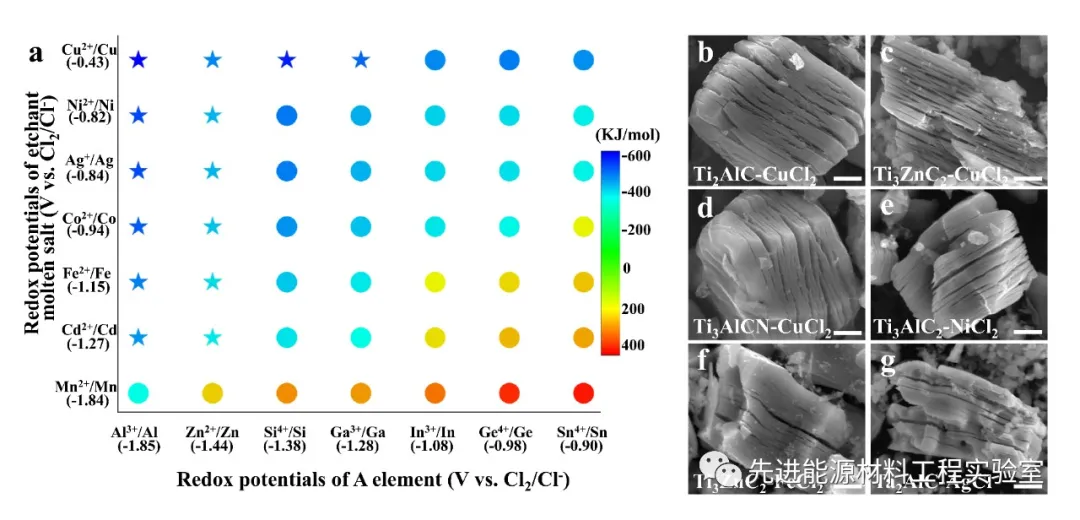
Fig. 2 The left picture shows the Gibbs free energy map of the oxidation-reduction potential / displacement reaction of cation and element A under high temperature molten salt environment. The right picture shows the MXene material finally obtained by the stripping system determined according to the map.

Figure 3 Characterization of the electrochemical performance of Ti 3 C 2 T x MXene in 1M LiPF 6 organic electrolyte.
Source of information: Advanced Energy Materials Engineering Laboratory







