¡¾Research Background¡¿
Since lithium metal (Li) has the advantages of low electrode potential and high specific capacity, it is the ultimate choice for the anode material of the next generation of high specific energy batteries. However, the uncontrollable dendrite growth and volume expansion caused by cycling in traditional electrolytes, and the induced low Coulomb efficiency (CE) have severely hindered its safety performance and cycle stability. Among them, the following two methods are considered to be the most effective solutions: (1) Structural engineering. Based on the lack of "primary" characteristics of metallic Li, a series of main structural skeletons are used to prepare composite lithium metal negative electrodes, thereby successfully promoting the cycle performance of the battery. However, due to the electronic conductivity of the rGO framework, Li is preferentially deposited on the outer surface and loses its corresponding protective effect; (2) Electrolyte engineering. Properly optimizing the electrolyte is the key way to establish a stable SEI and thereby passivate the highly active lithium metal. However, it is often difficult to achieve the result of dense deposition of Li through a single electrolyte improvement. Therefore, there is an urgent need for a synergistic method that combines an optimized "main body" structure with a high-performance electrolyte.
¡¾Achievement Introduction¡¿
Recently, Professor Cui Yi (corresponding author) of Stanford University in the United States reported on a combination of previously reported high-performance high-concentration electrolyte (LHCE) based on tris (trifluoroethoxy) methane (TFEO) and Si nanoparticles. The method of combining the main skeleton of reduced graphene oxide (SirGO). The advantage is that Si, as a nucleation seed for metal Li deposition, significantly inhibits the deposition of Li outside the framework, thereby helping to better protect Li deposition into the inside of the main structure. At the same time, the Li x Si alloy particles subsequently formed by controlling the cut-off voltage (0.4V) serve as the structure of the supporting skeleton, thereby maintaining the gap between the graphene layers, even after the Li is completely peeled off, the composite electrode will not appear large Volume fluctuations. More importantly, LHCE is conducive to building a more stable SEI. Based on the above advantages, the constructed Li || SirGO half-cell can steadily cycle more than 600 cycles with 99.4% CE. In addition, in the Li-SirGo || NMC532 full battery with N / P = 4, 380 cycles with negligible capacity attenuation can be performed. Therefore, this work not only optimizes the main structure of the Li metal anode, but also combines a combination of the most advanced electrolytes to obtain excellent battery electrochemical performance. The related research result "Improving Lithium Metal Composite Anodes with Seeding and Pillaring Effects of Silicon Nanoparticles" was published on ACS Nano .
¡¾core content¡¿
1. Comparison of Li deposition behavior
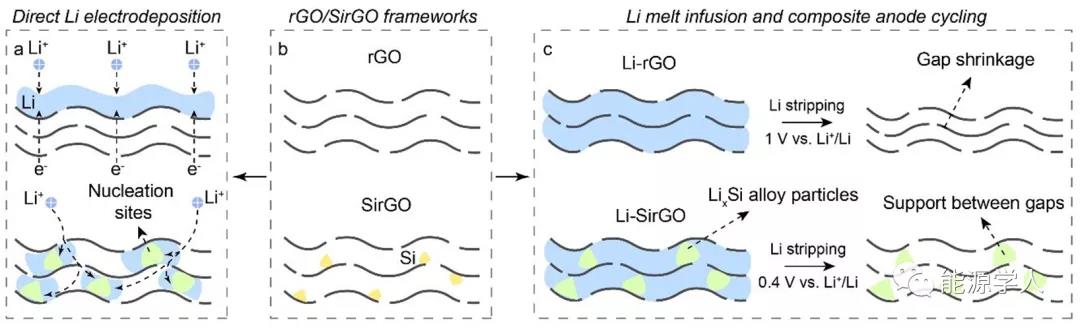
Figure 1. Comparison of the Li deposition behavior of SirGO and traditional rGO. (A) Li is preferentially deposited inside SirGO. On the contrary, Li preferentially deposits on the top of rGO; (b) Schematic diagrams of rGO and SirGO; (c) By controlling the cut-off voltage (0.4V), the Li x Si particles of the composite electrode are kept unchanged during the cycle , thereby achieving no change in electrode thickness .
Second, the preparation process of the composite electrode
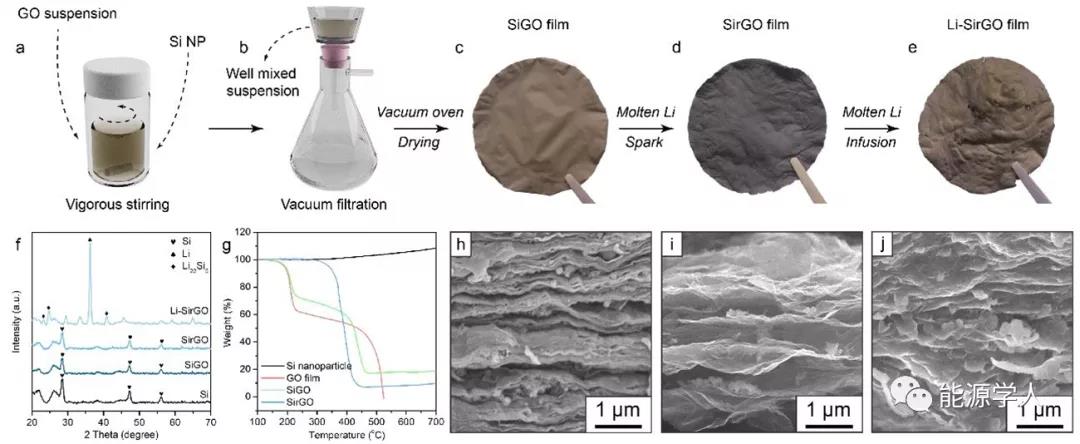
Figure 2a-e shows the synthesis process of Li-SirGO composite negative electrode. In turn, Si nanoparticles with a diameter of about 100 nm were uniformly dispersed in the GO solution through vigorous stirring and ultrasonic treatment, and then a Li-SirGO composite lithium metal anode was obtained by vacuum filtration, spark reaction, and molten Li injection. It can also be clearly seen from the corresponding SEM images that SirGO has a larger layer spacing and can embed more Si nanoparticles. At the same time, after the injection of molten lithium, the layers are filled with metallic lithium, and the particles accompanied by the Li x Si alloy morphology can also be confirmed by the corresponding XRD pattern.
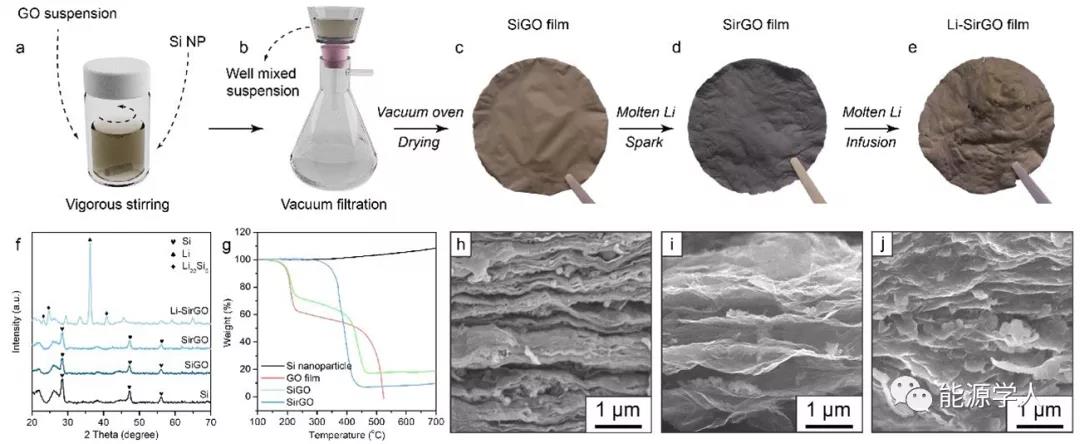
Figure 2. Preparation and characterization of Li-rGO composite electrode. (Ae) Preparation of SirGO by vacuum filtration and injection of molten Li to construct Li-SirGO composite anode; (f) XRD pattern; (g) thermogravimetric analysis (TGA) results; (hj) SiGO (h), SirGO (i) And SEM images of Li-SirGO.
3. The working mechanism of SirGO
The strategy of using intercalated Si nanoparticles as nucleation seeds for metal Li deposition successfully avoids the fact that Li is preferentially deposited on the surface of rGO. With the presence of Si nanoparticles between graphene layers, Li first forms an alloy with Si, and then lithium metal deposition occurs. Based on this working mechanism, it can be seen from the SEM image of FIG. 3 that SirGO modified with Si can preferentially deposit metal Li inside the framework, on the contrary, most of the surface of unmodified rGO appears Li, which constitutes Li- The SirGO composite electrode also exhibits ultra-dense deposition behavior. At the same time, after the Li-SirGO Li is stripped (cutoff voltage is 0.4V), it shows uniform distribution of Li x Si alloy particles.
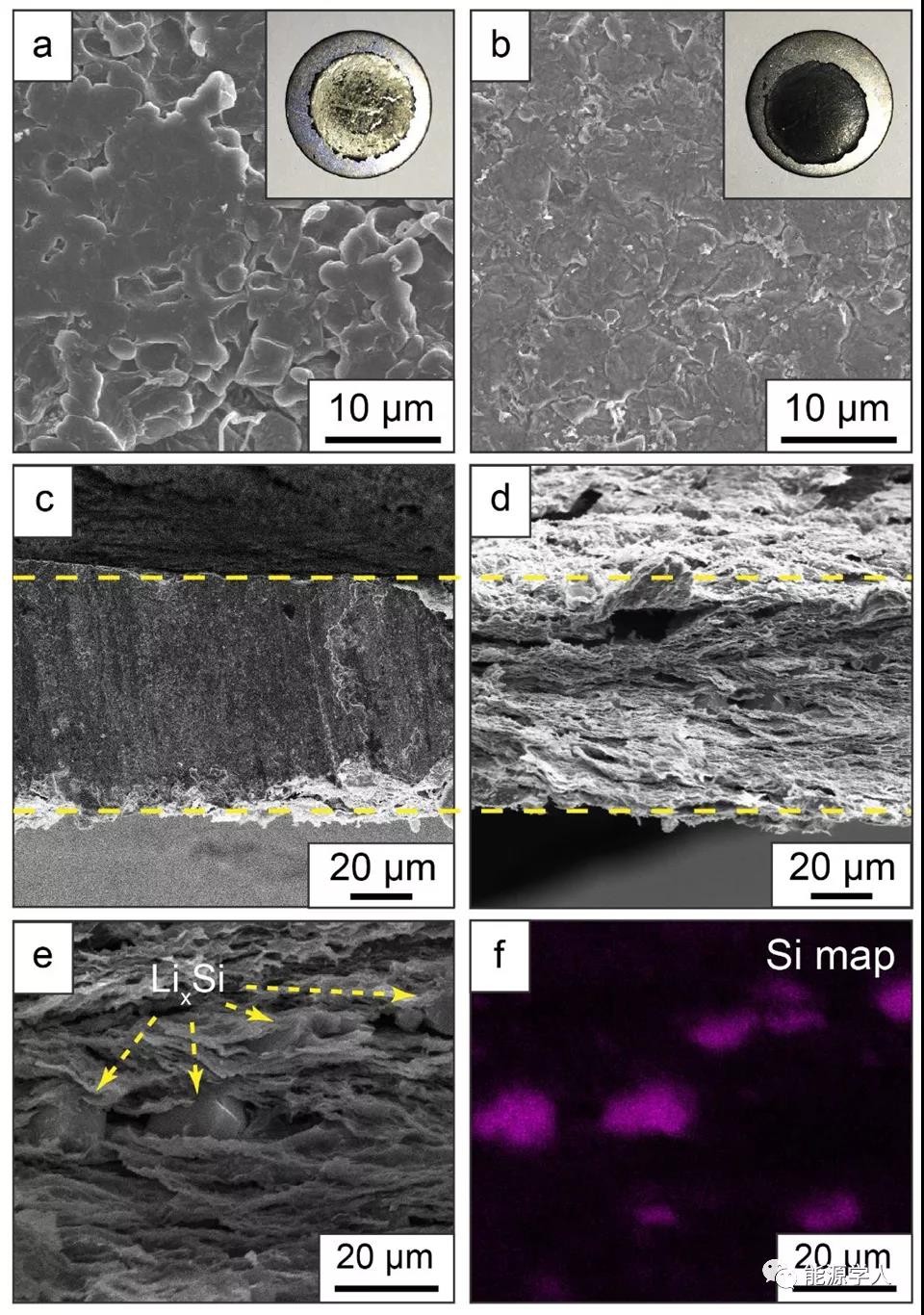
Figure 3. The working mechanism of the SirGO framework. (A, b) The morphology of SirGO and rGO after depositing 1 mAh cm -2 lithium at a current density of 1 mA cm -2 ; (c, d) Li-SirGO recombination before and after stripping metal Li to 0.4V SEM image of the cross section of the electrode; (e) SEM image of the enlarged area in (d); (f) the corresponding EDS spectrum.
4. The half-cell performance of SirGO
In order to prove the influence of the embedding of Si nanoparticles on the electrochemical performance of rGO, CE was tested under the conditions of 1 mA cm -2 and 1 mAh cm -2 by assembling half-cells . In contrast, SirGO, which also uses 1 M LiFSI / DME-TFEO as the electrolyte, can exhibit a higher and stable CE. It is more noteworthy that when the stripping voltage is 1.5V, the CE realized by SirGO has The large fluctuations are mainly due to the fact that some Li x Si particles do not have complete electronic contact with the framework, resulting in a one-time activation process; and the high cut-off voltage causes Li in Li x Si to be extracted, making the electrode structure unable to be Very well maintained. Therefore, the cut-off voltage of each cycle is set to 0.4 V, so that Li in Li x Si is not extracted, thereby improving the stability of CE. In addition, the symmetrical battery and impedance were further tested, and the performance has been greatly improved compared to before the modification.
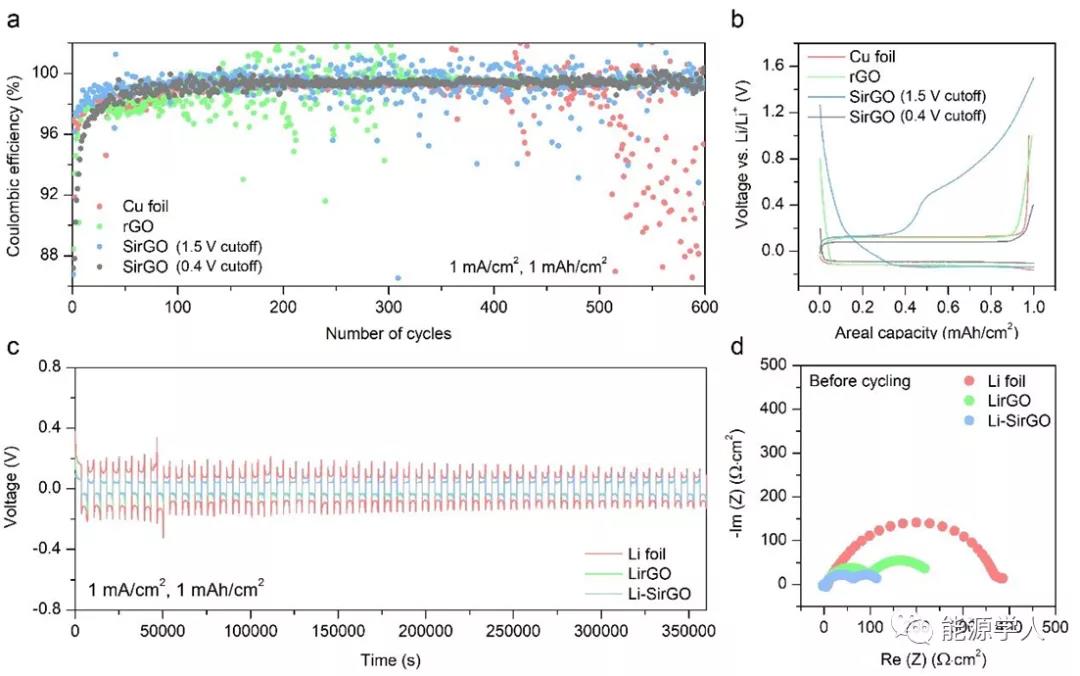
Figure 4. CE measurement and symmetrical battery cycle performance. (A) CE with cut-off voltages of 0.4V and 1.5V, respectively; (b) Corresponding stripping / deposition voltage curves; (c) Cycling performance of symmetrical cells; (d) Impedance measurement results of symmetrical cells before cycling.
5. Cycling performance of full battery
Further improvement in electrochemical performance was confirmed in the full-cell test. Use Li foil, Li-rGO and Li-SirGO (~ 10mAh cm -2 ) and NMC532 positive electrode (~ 2.5mAh cm -2 ) to assemble a full battery, and at 60 ¦Ìl of 1 M LiPF 6 / EC-DEC-FEC- Both VC and 1 M LiFSI / DME-TFEO electrolytes can show a significant improvement in cycle performance. These composite lithium metal anodes can not only further enhance the chargeability, but also further reduce the overpotential in the cycle.
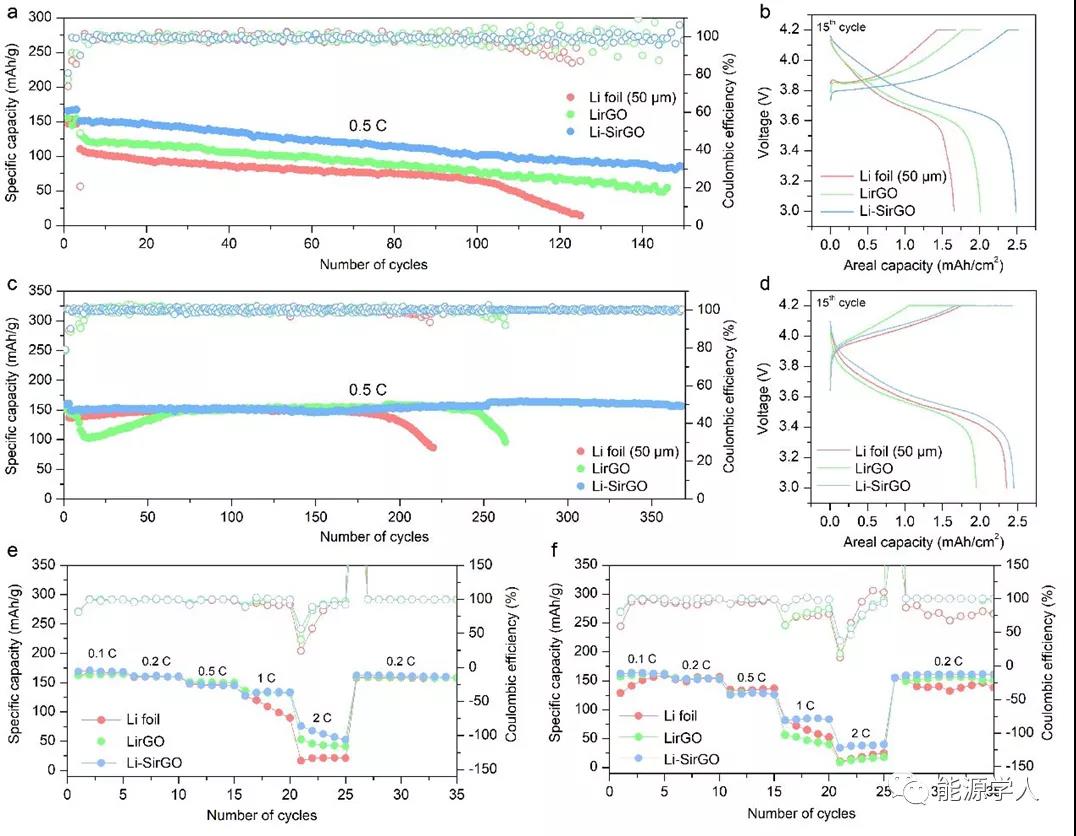
Figure 5. Full battery cycle performance. (A, b, e) Long-cycle performance, corresponding charge-discharge curve and rate performance of a full battery composed of NMC532 positive electrode and different negative electrodes in 1M LiPF 6 / EC-DEC-FEC-VC electrolyte; (c , d, f) Long-cycle performance, corresponding charge-discharge curve and rate performance of a full battery composed of NMC532 positive electrode and different negative electrodes in 1 M LiFSI / DME-TFEO electrolyte
¡¾Conclusion Outlook¡¿
In short, the authors further optimized the electrochemical performance of lithium metal anodes with rGO as the "host" frame by embedding Si nanoparticles between the graphene layers. This is mainly based on: (1) Even without pre-stored Li, Si can also act as a nucleation seed to cause Li to nucleate inside the rGO framework; (2) The voltage during the cycle is lower than 0.4V, thus ensuring Li x Si particles are always kept inside the skeleton, and there is no volume fluctuation. In addition to the electrode structure design, the composite negative electrode, the Li-compatible electrolyte and the NMC532 positive electrode are matched and assembled into a full battery, and a stable cycle of 380 cycles is achieved under the condition of limited Li source. Therefore, the strategy of combining electrolyte engineering with a three-dimensional composite negative electrode achieves higher cycle efficiency and rate performance. At the same time, it also proves the importance of combining various improvement methods to improve the performance of lithium metal anodes, and will accelerate the commercialization of high-energy density lithium metal batteries in the future.
Hansen Wang, Xia Cao, Hanke Gu, Yayuan Liu, Yanbin Li, Zewen Zhang, William Huang, Hongxia Wang, Jiangyan Wang, Wu Xu, Ji-Guang Zhang, Yi Cui *, Improving Lithium Metal Composite Anodes with Seeding and Pillaring Effects of Silicon Nanoparticles, ACS Nano , 2020, DOI: 10.1021 / acsnano.0c00184
Source of information: Energy Scholar









