【Highlights of this article】
(1) The author proposes a simple method to directly convert a two-dimensional T3C2Tx-MXene nanosheet into a three-dimensional carbon-coated MXene structure.
(2) Dopamine is synthesized by self-polymerization and carbonization on the surface of T3C2Tx nanosheets.
(3) The three-dimensional carbon-coated structure can protect T3C2Tx from air oxidation and structural aggregation.
(4) As a negative electrode material for lithium ion batteries and sodium ion batteries, it has high capacity and excellent rate performance.
【Key words】
MXene, carbon layer, white fungus structure, lithium ion battery, sodium ion battery
【Introduction】
Mxene has become a promising energy storage device in recent years due to its high-capacity capacitance and stable energy distribution. However, Mxene is prone to surface oxidation and interlayer redeposition, which affects its practical application in the field of energy storage.
This paper presents a simple method to directly convert 2D T 3 C 2 T x Mxene nanosheets into 3D carbon-coated T 3 C 2 T x structures. Nano-hybrid materials were prepared by self-polymerization of dopamine on the surface of MXene, followed by freeze drying and carbonization. The self-polymerization of dopamine not only promoted the transformation of the 2D-T 3 C 2 T x sheet to the 3D white fungus-like structure, but the subsequent carbonization led to the complete coverage of a thin carbon coating, which protected the structure Effects of air oxidation and structural aggregation. When used as a negative electrode material for lithium and sodium ion batteries, fast charge transfer, ultra-high capacity, excellent rate performance, and long cycle are achieved.

【Background Introduction】
1. Introduction to MXene
MXenes is a recently introduced group of two-dimensional materials, which has attracted great attention in the energy industry due to its striking physical and chemical properties. These transition metal carbides or nitrides represented by M n + 1 X n T x can be easily synthesized by etching the ‘A‘ layer from their ternary precursor (ie, M n + 1 AX n phase), where ‘M‘ is an early transition metal, while ‘A‘ is an element in groups 13 to 14 of the periodic table, X is C or N, N = 1-4, "T x " stands for surface termination, such as -OH, -O, -F and -Cl.
2. Features and application prospects of MXene
The two-dimensional layered surface area, high hydrophilicity and adjustable surface functionality make Mxene a versatile material with the characteristics required for various applications. Unlike graphene, Mxene‘s chemical diversity makes it a potential material for various applications. For example, the terminated Cr 2 CT x and Cr 2 NT x are known for their antiferromagnetic properties, while Cr 2 NO 2 can only exhibit ferromagnetic properties, while Mo-containing Mxene explicitly shows semiconductor-like characteristic.
Among the many available Mxene, Ti 3 C 2 T x is one of the most common and widely studied members. Graphene-like properties, coupled with high hydrophilicity and polar surface termination, make Ti 3 C 2 T x a good candidate for energy storage, catalysis, electromagnetic interference shielding, separation membranes, and sensors.
Contrary to graphene, Ti 3 C 2 T x and its hybrid derivatives are known for their pseudocapacitor-enhanced bulk capacitors, making them supercapacitors, lithium / sodium ion batteries, lithium-sulfur batteries, and lithium metals Promising materials in the field of batteries. For a lithium ion battery, of Ti . 3 C 2 T X theoretical lithium diffusion barrier (0.07 eV) relatively lower than that of graphite carbon (0.3 V), which is of Ti . 3 C 2 T X provides support for fast transfer of lithium ions and higher The ability to rate performance.
3. MXene‘s inherent shortcomings and improvement strategies
Now, although of Ti . 3 C 2 T X group having negative attractive energy distribution, two-dimensional materials due to various ubiquitous Van der Waals interactions, of Ti . 3 C 2 T X group is sensitive then deposited on the negative electrode layer. Such surface accumulation inhibits the accessibility of electrolytic ions, and at the same time reduces the charge transfer path, thereby damaging the true potential of MXene as a battery negative electrode material. In addition, Ti 3 C 2 T x -MXene is easily oxidized, resulting in complete conversion of TC bonds into TiO 2 particles and carbon, which seriously affects its energy storage capacity.
To overcome the above bottlenecks, the author considered various strategies, including the use of templates, self-assembly, and mechanical shearing. In recent methods, converting 2D MXene slices to 3D architecture has also proven effective. Under this background, Xiu et al. Proposed the three-dimensional hierarchical structure of MXene and obtained it by a simple spray drying method. The three-dimensional structure with no aggregation network recognizes higher surface area and higher conductivity, so that the constructed anode shows relatively superior performance.
Similarly, Gogotsi et al. Reported the MXene-based hollow sphere film prepared by the PMMA template method. Compared with two-dimensional films, the performance in SIBs has been improved. The integration of MXenes with traditional electroactive materials such as carbon nanotubes, conductive polymers and graphene is also considered a viable option, because the juxtaposition of these two active ingredients can mutually promote each other in a complementary manner.
In a recent study, Ren et al. Introduced the use of exposed active centers and shortened Li + diffusion paths to prepare MXene / CNTs porous membranes, which showed stronger capacity and higher rate performance in LIBs. Although converting 2D-MXene to 3D architecture may overcome the restacking problem, before MXene is conceived for practical application of LIBs, controlling undesired oxidation is another challenge that needs to be addressed. Thus, a simple and effective design strategy is essential, without impairing the policy allows of Ti . 3 C 2 T X under the integrity of the composition and stability inherent in the case of the-2D of Ti . 3 C 2 T X easy Convert to a 3D architecture with exposed surfaces.
【core content】
Recently, the team of Professor Xu Bin of Beijing University of Chemical Technology proposed a simple and effective strategy to obtain a new type of Tremella-like carbon-coated Ti 3 C 2 T x (T-MXene @ C) three-dimensional structure, which is used as a Effective negative electrode material. Using inert gas carbonization, dopamineself-polymerizeson pure Ti 3 C 2 T x nanosheets, resulting in a unique structure. The self-polymerization of dopamine promotes thetransformationof Ti 3 C 2 T x to a white fungus-like 3D structure. After carbonization, a thin carbon film is formed, which protects the exposed surface and prevents air oxidation and structural aggregation.
In order to fully understand the potential of T-MXene @ C, the authors performed competitive electrochemical measurements on freeze-dried Ti 3 C 2 T x (f-MXene) and heat-treated Ti 3 C 2 T x (h-MXene). Among them, the 3D structure (T-MXene @ C) with exposed and stable surface can support faster charge transfer, ultra-high capacity, and superior rate when used as negative materials for lithium (LIBs) and sodium ion batteries (SIBs) Performance and extended cyclic stability.
Overall, the relatively superior performance of T-MXene @ C makes it an ideal material for energy applications, while simultaneously controlling the morphology and stability of Ti 3 C 2 T x is to further predict MXene in energy storage devices It has excellent reciprocity with graphene.

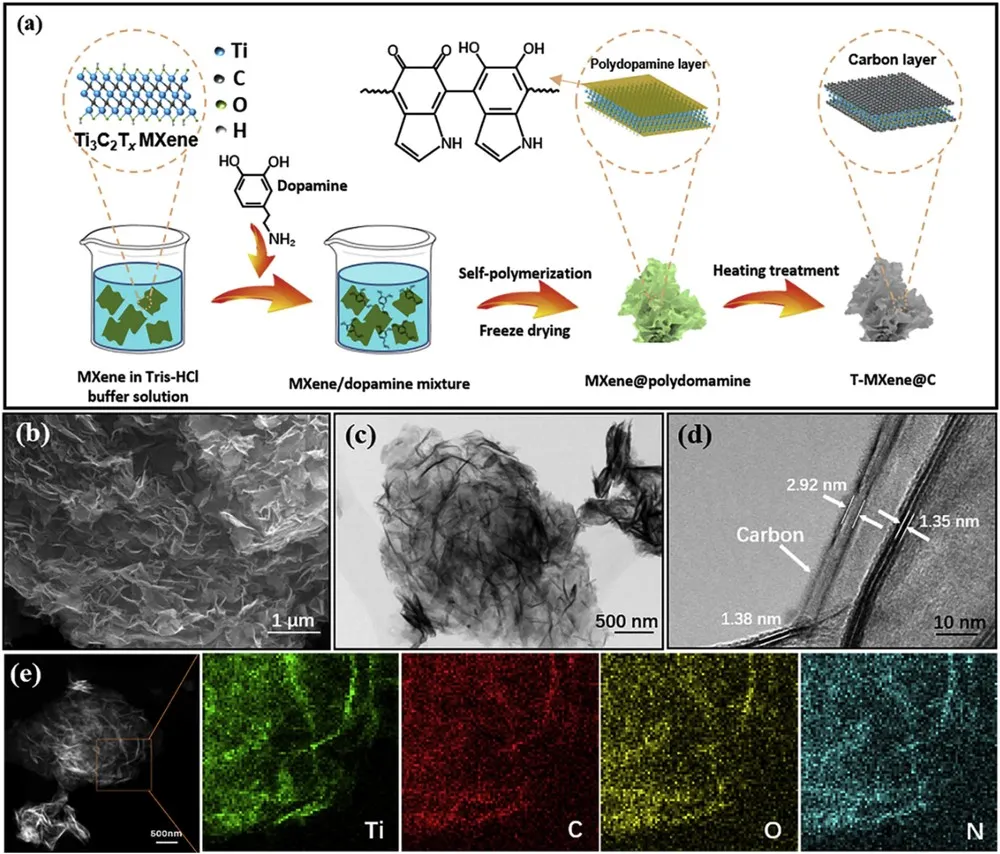
Figure 1. Schematic diagram and characterization of material synthesis
(a) Schematic diagram generalizing the preparation of T-MXene @ C. (b) SEM image, (c) Low-magnification TEM, (d) High-magnification TEM and (e) STEM images with representative EDS mapping indicating the distribution of Ti, C, O and N elements of the T-MXene @ C.

Figure 3. Comparison of lithium storage performance between T-MXene @ C, f-MXene and h-MXene
Lithium-ion storage study of T-MXene @ C. (A) CV profiles in the potential window of 0.01–3 V at a scan rate of 0.1 mV s−1, (b) initial three charge / discharge curves at a current rate of 0.2 C, (c) cycling performance at a current rate of 0.2 C, (d) rate performance of T-MXene @ C in comparison to f-MXene, and h-MXene, (e) long-term cycling performance (600 cycles) of the T-MXene @ C at 2 C (1C = 320 mA g−1).

Figure 5. Comparison of sodium storage performance between T-MXene @ C, f-MXene and h-MXene
Fig. 5. Sodium-ion storage behaviors of T-MXene @ C in comparison to f-MXene, and h-MXene. (A) CV profiles of T-MXene @ C measured within the potential window of 0.01–3 V at a scan rate of 0.1 mV s−1, (b) corresponding competitive cycling performance at a fixed current density of 0.05 A g−1, (c) rate performance at different current densities, (d) long-term cycling performance of T-MXene @C at 1 A g−1 with 3000 cycles, (e) the Na + diffusion coefficients estimated from the GITT potential profiles, (f) CV curves for T-MXene @ C at different scan rates, (g) the plot of corresponding current log ( i ) and log ( v ), (h) bar-graph depicting capacitive and diffusion controlled current contribution when scan rate changes from 0.2 to 10 mVs−1.
Source of information: Scientific Materials Station
Disclaimer: Purely academic, non-commercial use, if there is any infringement, please contact us immediately, we will delete it as soon as possible to protect the intellectual property of the original author








