已传文件:photo/20208248458877.png
Professor Xiaogang Peng (Zhejiang University)
1. J. Am. Chem. Soc. Realize oxygen-stable photoluminescence by deionizing CdSe/CdS core/shell quantum dots
The influence of oxygen on the photoluminescence properties of quantum dots can be regarded as a chemical reaction between quantum dots and oxygen, and how to achieve stable photoluminescence properties of oxygen is of great significance. The research group of Professor Xiaogang Peng of Zhejiang University systematically and quantitatively studied the effects of oxygen on quantum dots by using the clear spectral characteristics of high-quality CdSe/CdS core/shell quantum dots at the single point and overall (on the substrate and in solution) levels. The effect of photoluminescence (PL) reveals a unified and simple image. During the photoexcitation process, when there is enough oxygen in the system, various forms of core/shell quantum dots will be deionized in time, returning from the inefficient triplet state of light generation to the efficient single exciton state, and the oxygen is reduced to Superoxide radicals. Under a certain excitation power, the rates of spontaneous deionization channels and photodeionization channels both increase with the increase of oxygen pressure, but the photoionization of quantum dots is hardly affected by oxygen pressure. Although oxygen-stable photoluminescence has been found in both CdSe ordinary nuclear quantum dots and CdSe/CdS core/shell quantum dots, only irreversible photo-corrosion has been observed in CdSe ordinary nuclear quantum dots, which indicates a high-quality epitaxial shell pair The importance of quantum dots in various applications. In general, for basic research and technical applications, quantum dots with high-quality epitaxial shells and near-ideal photoluminescence properties are necessary to avoid the complex and destructive effects of oxygen. [1] Related research was published on J. Am. Chem. Soc. entitled "Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization".
Figure 1: Realization of photoluminescence of quantum dots with oxygen stability

2. Nature Communications‘ electrochemically stable ligands bridge the photoluminescence-electroluminescence gap of quantum dots
Colloidal quantum dots are promising emitters used in quantum dot light-emitting diodes. Although photoluminescent quantum dots with high efficiency, stability and high color purity have been synthesized, the unbalanced charge injection and/or interface exciton quenching in the device have inherited its excellent luminescence performance in light-emitting diodes. There are certain challenges. The research group of Professor Xiaogang Peng from Zhejiang University reported on core/shell quantum dots with various combinations of shell and surface ligands, and developed a strategy of applying electrochemically inert ligands to quantum dots with excellent luminescence properties to bridge them The photoluminescence-electroluminescence gap. This material design principle is usually used to improve the electroluminescence efficiency and the life of light-emitting diodes, so that red light-emitting diodes (at 1000 cd m-2, T95> 3800 h) and blue-emitting light-emitting diodes (at 100 cd m- Two locations with T50> 10000 h) have reached an unprecedented working life. This research provides important guidance for the application of quantum dots in optoelectronic and electronic devices. [2] Related research was published on Nature Communications with the title "Electrochemically-stable ligands bridge the photoluminescence-electroluminescence gap of quantum dots".
Figure 2: Characterization of PL and EL characteristics of CdSe/CdS-Cd(RCOO)2 and CdSe/CdS-RNH2 QD

3. Advanced Functional Materials uses high-performance quantum dot light-emitting diodes with a high and stable work function NiOx hole injection layer
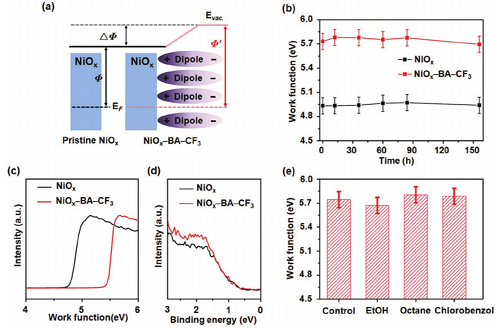
In quantum dot light-emitting diodes, the oxide film prepared by the solution method is actively used as the hole injection layer to improve the working stability. However, the device performance is largely limited by the hole injection efficiency at the interface between the oxide hole injection layer and the high ionization potential organic hole transport layer. The research group of Professor Xiaogang Peng of Zhejiang University reported a simple and easy surface modification strategy to prepare NiOx films with high and stable work function (approximately 5.7 eV) by solution method. The driving voltages of light-emitting diodes based on the surface-modified NiOx hole injection layer are 2.1 V and 3.3 V, respectively, reaching 1000 and 10000 CD•m-2, which are in all solution-processed light-emitting diodes and vacuum-deposited organic light-emitting diodes Both are the lowest. The T95 working life of this device is about 2500 h when the initial brightness is 1000 CD•m-2, which meets the commercial requirements of display applications. This research shows the potential of the solution-processed oxide hole injection layer to achieve high-efficiency driving and long-life LEDs. [3] Related research was published on Advanced Functional Materials with the title of "High-Performance Quantum-Dot Light-Emitting Diodes Using NiOx Hole-Injection Layers with a High and Stable Work Function".
Figure 3: Characterization of NiOx-BA-CF3
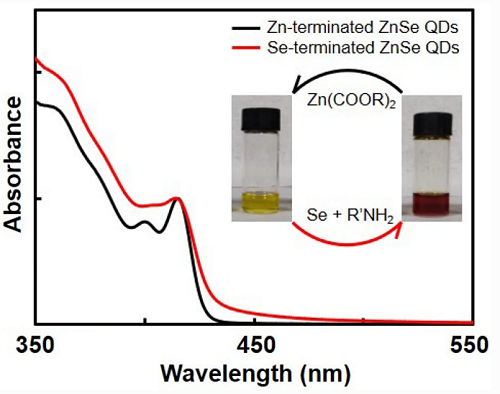
4. The surface and intrinsic contribution of Nano Research ZnSe quantum dots‘ extinction properties
In addition to the commonly observed photoluminescence quenching caused by anion surface sites, Se-ZnSe quantum dots also clearly show the characteristics of the Se surface state in their ultraviolet-visible absorption spectrum. The research group of Professor Xiaogang Peng from Zhejiang University reported on the extinction properties of ZnSe quantum dots with Se surface or Zn surface (Se-ZnSe or Zn-ZnSe quantum dots) as the termination point. Similar to most quantum dots reported in the literature, monodisperse Zn-ZnSe quantum dots show obvious absorption characteristics and blue-shifted but steep absorption edges relative to the overall band gap. However, for monodisperse Se-ZnSe quantum dots, all the absorption characteristics are smeared, and an energy window with a low-energy tail extending below the ZnSe band gap is found. With the increase of the size of quantum dots, the periodic growth of ZnSe quantum dots causes the surface to change from Zn end to Se end, which confirms that the specific absorption characteristics are repeatable between the two quantum dots. Although the unit extinction coefficient of Se-ZnSe quantum dots is always greater than that of Zn-ZnSe quantum dots of the same size, they are all close to the same volume limit. In addition to the contribution of the lattice, the extinction coefficient of each nanocrystal of Zn-ZnSe quantum dots has an exponential relationship with their size, which is expected to enhance the quantum confinement of electron-hole wave function overlap. For Se-ZnSe quantum dots, the extinction coefficient of each nanocrystal has a third term, which is proportional to the square of the size of the quantum dot and is consistent with the surface contribution. This research provides new ideas for exploring the surface and intrinsic contribution of quantum dots‘ extinction properties. [4] Related research was published on Nano Research with the title "Surface and intrinsic contributions to extinction properties of ZnSe quantum dots".
Figure 4: Characterization of UV absorption of ZnSe quantum dots
Moungi Bawendi (Massachusetts Institute of Technology)
5. J. Am. Chem. Soc. Scalable synthesis of InAs quantum dots mediated by indium redox chemistry
Next-generation optoelectronic applications centered on near-infrared (NIR) and short-wave infrared (SWIR) wavelengths require high-quality materials. Among these materials, colloidal InAs quantum dots (QDs), as a kind of infrared active candidate materials for biological imaging, lighting and sensing applications, have significant developments in their optical properties, but the synthesis of InAs quantum dots still often relies on dangerous , Precursors that are not commercially available. The research group of Professor Moungi G. Bawendi from MIT reported a simple single-shot thermal injection process with In(I)Cl as the main precursor, and as a reducing agent and indium source at the same time, In(I)Cl successfully combined with three( Amino) arsenic precursor reacted to synthesize colloidal InAs QDs quantitatively and gram-level. By adjusting the reaction temperature, an InAs nucleus with the first exciton absorption characteristic is produced, and its absorption range is 700−1400 nm. The dynamic disproportionation balance between In(I), In(0) and In(III) provides additional flexibility for precursor selection. The CdSe shell layer grown on the prepared core enhances their optical properties, provides particles with a central emission wavelength between 1000 and 1500 nm, and the entire narrow photoluminescence has a full width at half maximum (FWHM) of approximately 120 meV. The simplicity, scalability and tunability of the disclosed precursor platform of the institute is expected to stimulate further research on In-based colloidal quantum dots. [5] Related research was published on J. Am. Chem. Soc. with the title "Scalable Synthesis of InAs Quantum Dots Mediated through Indium Redox Chemistry".
Figure 5: Schematic diagram of the route for preparing high-quality InAs QDs

6. Coherent single photon emission of Science colloidal lead halide perovskite quantum dots
Chemically synthesized colloidal semiconductor quantum dots have been proposed as scalable and tunable single emitters in quantum optics, but they usually suffer from prohibitive incoherent emission. The research group of Professor Moungi G. Bawendi from the Massachusetts Institute of Technology reported that single colloidal lead halide perovskite quantum dots (PQDs) exhibit high-efficiency single-photon emission with an optical coherence time of up to 80 ps, which accounts for the equivalent of 210 ps radiation lifetime. Part. These measurements indicate that PQDs should be explored as part of the source of indistinguishable single-photon and entangled photon pairs. The research results provide a starting point for the rational design of lead halide perovskite-based quantum emitters. The proposed quantum dot emitters have fast emission, wide-spectrum tunable and scalable production capacity, and benefit from the existing colloid Materially proven hybrid integration of nanophotonic components. [6] Related research was published on Science with the title of "Coherent single-photon emission from colloidal lead halide perovskite quantum dots".
Figure 6: Schematic diagram of characterization of cesium lead PQD
7. Small zinc thiocyanate can realize bright copper-deficient Cu-In S / ZnS quantum dots
Indium copper sulfide (CIS) colloidal quantum dots (QDs) have good commercial prospects in quantum dot-based optical applications, such as colloidal photocatalysts or luminescent solar concentrators (LSCs). CIS quantum dots with good photoluminescence quantum yields (PLQYs) and tunable emission wavelength have been reported previously. However, the development of understanding and control of the growth of electron-passivated inorganic shells will help to further improve the photophysical properties of CIS quantum dots. In order to improve the optical performance of CIS quantum dots, the focus is to grow the inorganic shell layer through the metal-carboxylate/alkanethiol decomposition reaction. The research group of Professor Moungi G. Bawendi from the Massachusetts Institute of Technology studied the role of Zn-carboxylate and Zn-thiolate in forming a ZnS shell on copper-deficient CIS (CDCIS) quantum dots, and obtained photoluminescence by using this principle. CDCIS/ZnS core-shell quantum dots with a quantum yield greater than 90%, and the mechanism of Zn-carboxylate/alkanethiol decomposition to generate ZnS shell is proposed. This provides ideas for studying the potential mechanism of quantum dot synthesis. [7] Related research was published on Small with the title of "Zinc Thiolate Enables Bright Cu-Deficient Cu-In-S/ZnS Quantum Dots".
Figure 7: Synthesis diagram of quantum dots and photoluminescence research

8. Small Methods High-efficiency translucent CsPbI3 quantum dot photovoltaic using graphene electrodes
Due to the tunability of the band gap, solution-processed perovskite quantum dots (QDs) are expected to be candidates for the preparation of translucent and tandem solar cells. The research group of Professor Moungi G. Bawendi of the Massachusetts Institute of Technology reported the synthesis of cesium lead triiodide (CsPbI3) quantum dots using a stable cubic phase, and the preparation of high-efficiency perovskite solar cells (PSCs) using ligand exchange technology. The research used chemical vapor deposition to grow a single layer of graphene and developed a dry process to transfer graphene to the top of the device. On this basis, a high-efficiency reverse PSC with high average visible light transmittance (AVT) is proposed. After optimization, PSCs based on silver and graphene electrodes were obtained, with power conversion efficiency (PCE) of 9.6% and 6.8%, respectively. In addition, by adjusting the thickness of the active layer, a PSC with a PCE of 4.95% and AVT of 53% can be obtained, indicating the potential of CsPbI3 quantum dots in the production of translucent devices suitable for windows. [8] Related research was published on Small Methods with the title "Efficient Semitransparent CsPbI3 Quantum Dots Photovoltaics Using a Graphene Electrode".
Figure 8: Characterization of CsPbI3 quantum dots

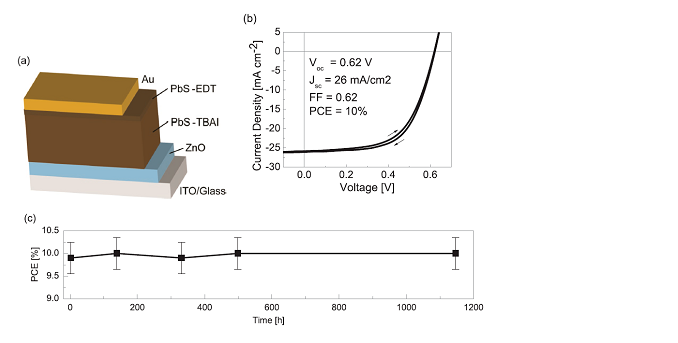
9. Advanced Sustainable Systems reduces the synthesis cost and waste toxicity of lead sulfide quantum dot ink photovoltaic
The use of lead sulfide (PbS) colloidal quantum dot (QD) film as the photosensitive layer in photovoltaic devices usually requires lead-based end-capped ligands (ie PbX2, X = Br, I) instead of natural QD ligands to obtain the best performance High-performance QD photovoltaic devices, however, this ligand replacement process usually requires additional solvents and toxic reagents. The research group of Professor Moungi G. Bawendi of the Massachusetts Institute of Technology reported a PbS QD photovoltaic preparation method using lead-free tetrabutylammonium iodide (TBAI) ligand source. This method has lower material requirements and toxicity. Under the conditions, a power conversion efficiency of 10% can be obtained, and the storage stability is above 1000 h. Compared with the previous scheme based on PbX2 to prepare the best QD PVs, the evaluation of the economic and toxicological benefits of this new ligand exchange scheme shows that the synthesis cost is reduced.
Figure 9: Schematic diagram of PbS QD prepared by tetrabutylammonium iodide photovoltaic
Pang Daiwen (Nankai University/Wuhan University)
10. Science China Chemistry uses quantum dots synthesized in cells to design microbubbles in situ for cell self-labeling
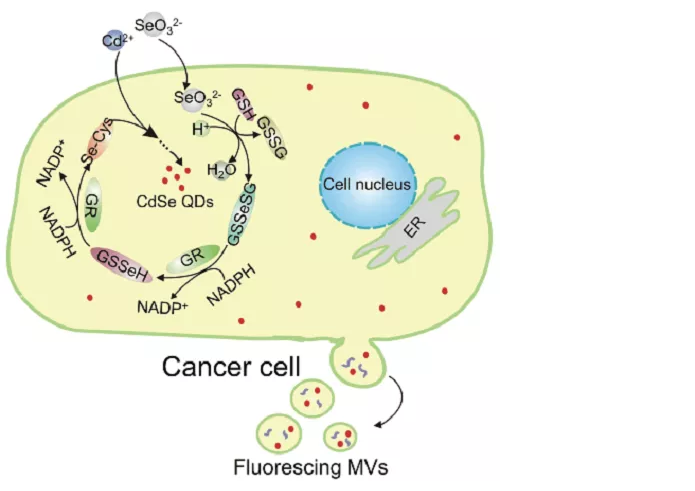
Cell-derived microvesicles (MVs) are secreted from almost all types of mammalian cells into the extracellular space, and play a key role in intercellular communication and the transport of biomolecules between cells. However, due to the limitations of existing labeling methods, the visualization and monitoring of MVs biological behavior is facing huge challenges. The research group of Professor Pang Daiwen of Nankai University/Wuhan University reported the first in the in-situ design of MVs secreted by living mammalian MCF-7 cells using fluorescent quantum dots (QDs) synthesized in cells during the formation of MVs. Examples. By carefully coupling the intracellular biochemical reactions and metabolic pathways, MCF-7 cells can be irradiated with fluorescent CdSe QDs biosynthesized in the cells. At the same time, QDs synthesized in cells can be wrapped in situ by MVS secreted by fluorescent cell plasma membrane, and the efficiency of labeling MVS is as high as 89.9%. The entire labeling process cleverly combines the synthesis of CdSe quantum dots designed for precise cell tuning and gentle in situ labeling through cell self-realization, which can be performed only after feeding the cells with appropriate chemicals, which is a structure or function It is not destructive, and is simpler and gentler than chemical coupling or indirect encapsulation with conventional fluorescent labels. [10] Related research was published in Science China Chemistry with the title of "Designer cell-self-implemented labeling of microvesicles in situ with the intracellular-synthesized quantum dots".
Figure 10: Intracellular synthesis of fluorescent QD in live MCF-7 cells and schematic diagram of microbubble labeling
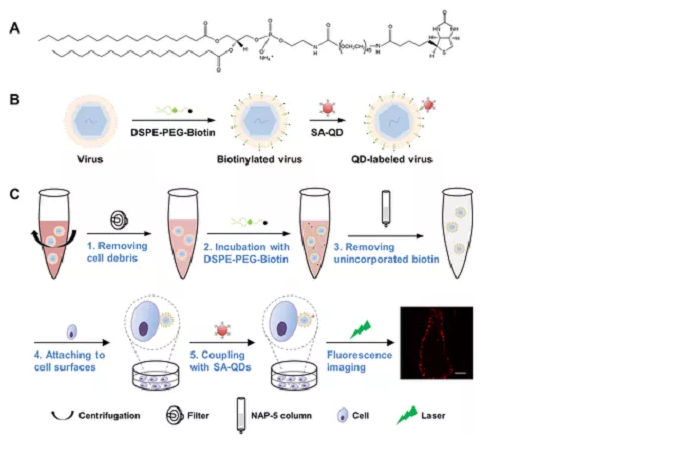
11. Lipid-specific labeling of enveloped viruses of mBio quantum dots for single virus tracking
Quantum dots (QDs) have optical characteristics such as very bright fluorescence, excellent light stability, narrow emission spectrum and optional colors. After labeling with quantum dots, a single molecule/virus can be imaged continuously for a long time, providing more detailed information than when labeling with other fluorophores. Although quantum dots are widely used to label proteins in single-molecule tracking studies, they are rarely used to study viral infections, mainly because of the lack of recognized labeling strategies. The research group of Professor Pang Daiwen of Nankai University/Wuhan University reported a general method for labeling enveloped viruses with quantum dots gently and easily. The lipid biotin conjugate is used to identify and label the viral plasma membrane, and the streptavidin-QD conjugate is used to illuminate them. This method can label enveloped viruses within 2 hours, with specificity and efficiency of 99% and 98%, respectively, and the complete morphology and inherent infectivity of the virus are retained. Using this QD labeling method, wild-type and mutant Japanese encephalitis viruses were labeled, and their infection in live Vero cells was tracked. It was found that the replacement of H144A and Q258A in the envelope protein did not affect the virus‘s intracellular transport. The lipid-specific QD labeling method described in this study provides a simple and practical tool that makes it easy to "observe" viruses and track their infection, which is conducive to the wide application of single virus tracking and the discovery of complex infection mechanisms . [11] The related research was published on mBio with the title of "Lipid-Specific Labeling of Enveloped Viruses with Quantum Dots for Single-Virus Tracking".
Figure 11: Schematic diagram of quantum dot labeled enveloped virus
references:
[1] Hu Z., Liu S., Qin H.*, Zhou J., Peng X.*, Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization. [J] J. Am. Chem. Soc . 2020, 142, 9, 4254-4264.
[2] Pu C., Dai X., Shu Y., Zhu M., Deng Y., Jin Y., Peng X.*, Electrochemically-stable ligands bridge the photoluminescence-electroluminescence gap of quantum dots. [J] Nature Communications 2020, 11, 937.
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately














