已传文件:photo/1600912363.png
Noble metals mainly refer to 8 metal elements including gold, silver and platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, platinum), among which gold is particularly important. Most of these metals have beautiful colors and strong chemical stability. Under normal conditions, they are not easy to chemically react with other chemicals. Precious metals with unique physical and chemical properties have been extensively studied and used in the fields of catalysts, conductors, medicines, etc. Due to their limited reserves and high cost, solutions to recover precious metals from water and waste have strong

economic attractiveness. Researchers have made great efforts in the recovery of precious metals. Many methods including biosorbents, cementation, adsorption, ion exchange, and solvent extraction have been developed. However, the efficiency of these recycling technologies is not satisfactory, and a large amount of secondary waste is usually generated due to the addition of chemical reagents for precipitation and reduction of metals. Therefore, there is an urgent need to develop a method that can recover precious metals from wastewater with low cost and good eco-friendliness, which not only has significant economic value, but also promotes sustainable development. Recently, separation membranes have received extensive attention due to their high efficiency, low energy consumption and simple operation. So far, membrane technology has been widely used in the fields of seawater desalination, water purification, precious metal recovery and gas separation. The basic technology of water purification membranes is usually divided into ultrafiltration, nanofiltration, reverse osmosis, nano hybrid membrane and electrodialysis. Nanofiltration membranes have shown the ability to selectively separate ions based on size and charge. The nanochannel membrane acts as a molecular sieve, preventing all molecules and ions with a hydration diameter larger than the channel diameter, and therefore has proven to have great potential for removing organic molecules and metal ions from water. The hydration radius of inorganic salts and organic

molecules sieved by the graphene oxide membrane is> 4.5Å. However, the hydration radius of most precious metal ions is less than 4.5 Å, so this limits the selectivity of filtration. Reducing the diameter of the nanochannel will hinder the flow of water molecules, so that due to the inherent permeability/repellency trade-off, it will be difficult to achieve high permeability at the same time without sacrificing separation efficiency. Therefore, it is important to prepare a membrane with both high permeability and high barrier rate, but it is also a difficult point. MXene is a two-dimensional inorganic compound in materials science. These materials consist of transition metal carbides, nitrides or carbonitrides with a thickness of several atomic layers. It was first reported in 2011 that due to the hydroxyl or terminal oxygen on the surface of MXene materials, they have the metal conductivity of transition metal carbides. For MXene membranes, the trade-off between permeability and repellency remains a huge challenge. In addition, MXene film has poor stability in aqueous media. Research results Recently, the research group of Professor Liu Chang from the Institute of Metal Research of the Chinese Academy of Sciences reported a sandwich structure of Ti3C2TxMXene/carbon nanotube composite film in which CNTs isolate and support the MXene sheet. The composite membrane shows an excellent ability to capture precious metal ions and has a high flux. The water permeability of the membrane reaches 437.6 L m –2 h –1 bar–1 (2.46×10 –18m2), which is about 202 times that of pure Ti3C2Tx, and can capture 99.8% of gold from a very low gold concentration of 20 ppm solution ( III). The ideal precious metal trapping ability of Ti3C2Tx-CNT film is due to the high redox activity of C-Ti-OH. This work provides a reference for the recovery of precious metal ions in wastewater. Related work was published on the internationally renowned journal ACS Applied Materials & Interfaces with the title "MXene-Carbon Nanotube Hybrid Membrane for Robust Recovery of Au from Trace-Level Solution". Micro-morphology characterization of Ti3C2Tx-CNT Ti3C2Tx MXene is prepared by selectively etching Al from MAX (Ti3AlC2) phase with LiF in hydrochloric acid. A certain amount of MXene slurry is added to deionized (DI) water, and then subjected to ultrasonic treatment and centrifugal separation to obtain MXene nanosheets. The Tyndall scattering effect in the prepared MXene colloid can be clearly observed. As shown in Figure 1a, a clear laser beam passes through, indicating that MXene has good dispersibility in water. The TEM images shown in Figure 1 c and d show that the exfoliated MXene nanosheets are very thin and uniform, which confirms their 2D nature.
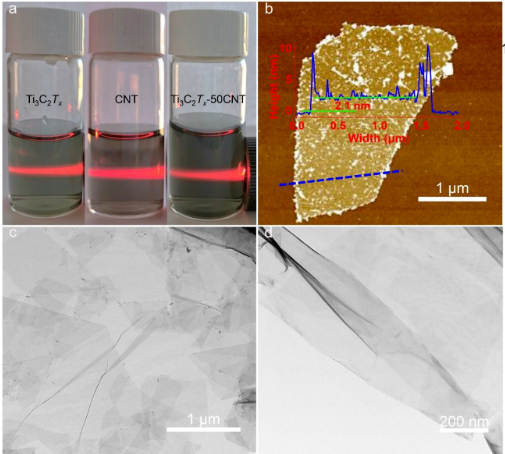
Figure 1 SEM and AFM characterization of Ti3C2Tx-CNT
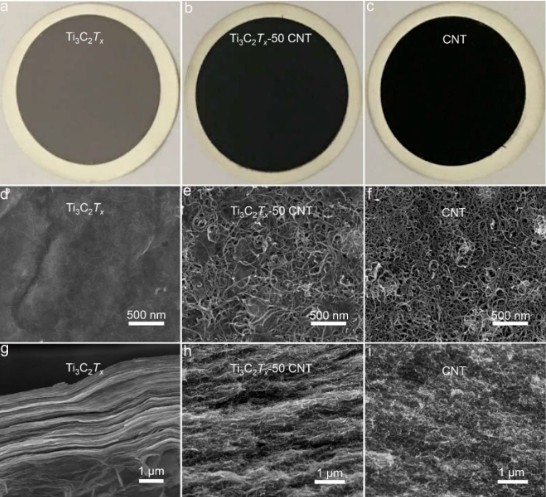
Figure 2. SEM characterization of Ti3C2Tx-CNT membrane permeability and separation performance The water permeability of pure MXene is extremely low (2.6 L m –2 h –1 bar –1 or 2.25 × 10 – 21 m 2), but in the composite membrane, specifically, the permeability of Ti3C2Tx-20 CNT increased to 30.5 L m –2 h –1 bar –1 (4.72 × 10 –20 m 2), which is 11.7 times that of pure Ti3C2Tx . Surprisingly, the permeability of Ti3C2Tx-80 CNT is as high as 674.7 L m –2 h –1 bar –1 (3.79 × 10 –18 m 2), which is about 260 times that of pure Ti3C2Tx MXene. The significant increase in permeability is attributed to the mesopores and macropores created by the addition of carbon nanotubes, which provide sufficient nanochannels for the rapid passage of water molecules. The separation performance of the sample with an initial concentration of 20 ppm of gold (III) in HAuCl4 is shown in Figure 3a. It can be seen that the Ti3C2Tx-CNT membrane exhibits high permeability and high separation efficiency.
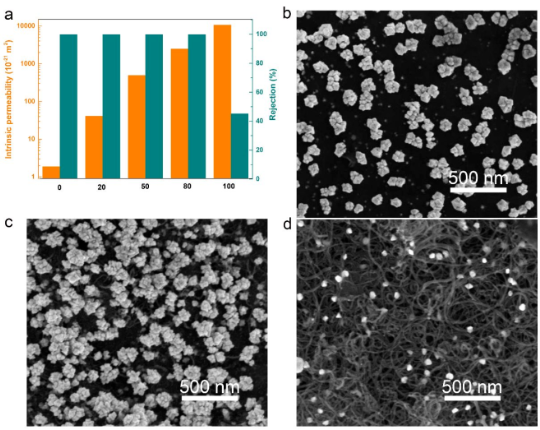
Figure 3. (a) Characterization of membrane permeability and barrier rate; (bd) SEM image separation mechanism study of Ti3C2Tx-Au, Ti3C2Tx-50, CNT-Au and CNT-Au films. The oxidation state of the terminal Ti atoms in Ti3C2Tx MXene is Unsaturated, which allows electrons to spontaneously donate it to the precious metal cation, reducing it to metal nanoparticles. Previous studies reported that the oxygen-containing functional groups on CNT can reduce gold (III) to gold (0). Our results show that the pure CNT film containing functional groups can indeed reduce gold (III) to gold (0); however, the reduction ability of carbon nanotubes is very limited, which indicates that the reduction ability of carbon nanotubes is far lower. In Ti3C2Tx, the surface reducibility of Ti3C2Tx terminated by -OH functional group is higher than that of Ti3C2Tx terminated by -O functional group, while the original Ti3C2Tx MXene has electronically unsaturated terminal Ti atoms. -OH can provide electrons, with the result that C-Ti-OH is oxidized to C-Ti-O and/or Ti-O by noble metal cations. Figure 4 XRD and XPS characterization
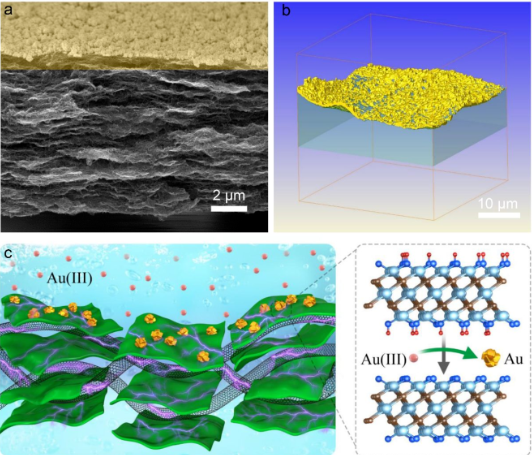
Figure 5 (a) Cross-sectional SEM image of Ti3C2Tx-50 CNT. (B) 3D XRT image of Ti3C2Tx-50 CNT-. (C) The schematic diagram shows the mechanism of the redox reaction. Summary: In summary, this article reports a simple method to prepare a highly stable Ti3C2Tx MXene-CNT composite membrane, which is used as a filter membrane for the recovery of precious metals from very low concentration solutions. The author proposed a redox reaction rejection mechanism and proved it. The C-Ti-OH of MXene is oxidized to C-Ti-O and/or Ti-O, and the noble metal cation is reduced to zero-valent noble metal. CNT prevents the agglomeration of the MXene layer, acts as an electron transfer, and enhances the stability of the composite membrane. As a result, the MXene-CNT membrane has an extremely high permeability of 437.6 L m –2h –1 bar –1 (2.46×10 –18 m2), and shows great advantages in capturing extremely low concentrations of precious metal ions. This work provides a new method for the design and preparation of membrane materials for the recovery of precious metals.
Information source: Frontiers of Polymer Science
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately









