
Microgels are gradually entering the field of tissue regeneration because they overcome the problems of traditional bulk/macroscopic hydrogels, such as limited cell-to-cell contact and cell-to-cell communication and low diffusion rate. Since minimally invasive surgery can enhance mass transfer and injection capabilities, these microgels are becoming a promising application method for bone regeneration. However, there is still a big gap in the understanding of how the composition of the gel matrix affects the cell response and overall tissue formation. There is still a big gap in the understanding of the conversion from the overall structure to the microgel structure. In response to this problem, the team of Professor Jessica E. Frith from Monash University, Australia Published a research paper entitled "Interplay of Hydrogel Composition and Geometry on Human Mesenchymal Stem Cell Osteogenesis" on Biomacromolecules. The authors prepared polyethylene glycol-based microgels containing gelatin and hyaluronic acid (HA) alone or at the same time, and evaluated the influence of the composition of the hydrogel on the osteogenic differentiation of encapsulated human bone marrow mesenchymal stem cells (hBMSCs) (figure 1).
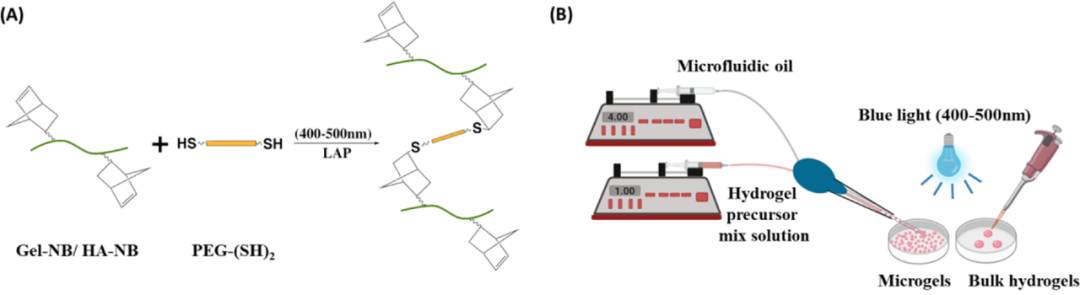
Figure 1 (A) Schematic diagram of the thiol-norbornene click reaction; (B) Schematic diagram of the preparation of bulk hydrogel and microgel
First, the author designed three different hydrogels containing thiol-functionalized PEG (PEG-(SH)2), and combined them with norbornane functionalized gelatin (Gel-NB) or norbornyl functionalized hyaluronic acid (HA -NB) or a mixed gel of Gel-NB and HA-NB. The photocrosslinking kinetics of the hydrogel was monitored by in-situ photorheology, and the results showed that, regardless of the composition of the hydrogel, the gel can quickly form within a few seconds of light exposure. From the stress-strain curve obtained from the compression test, it can be seen that the compressive moduli of Gel, Gel:HA and HA hydrogels are 5.2, 3.3 and 2.3 kPa, respectively, which are the same as the storage modulus. These data indicate that the hardness of the hydrogel shows a trend of Gel>Gel:HA>HA. Since hydrogels with high water content tend to be softer than hydrogels with low water content, the difference in hydrophilicity between HA and gelatin may also lead to differences in the mechanical properties of the hydrogels formed by them (Figure 2).
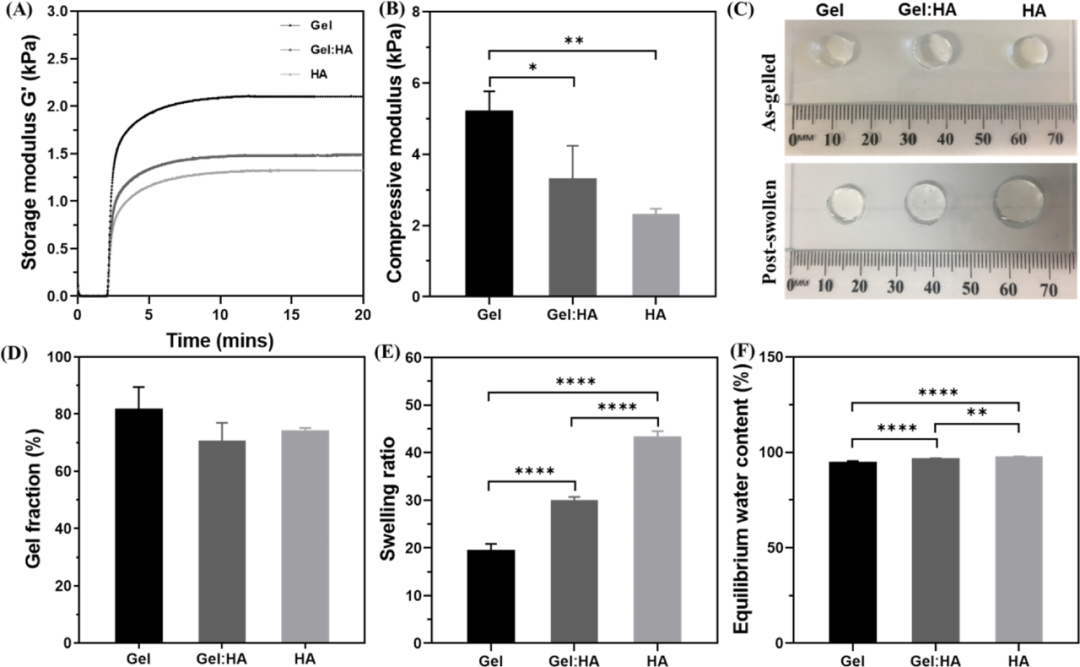
Figure 2 Gel, Gel: Characterization of HA and HA block hydrogels
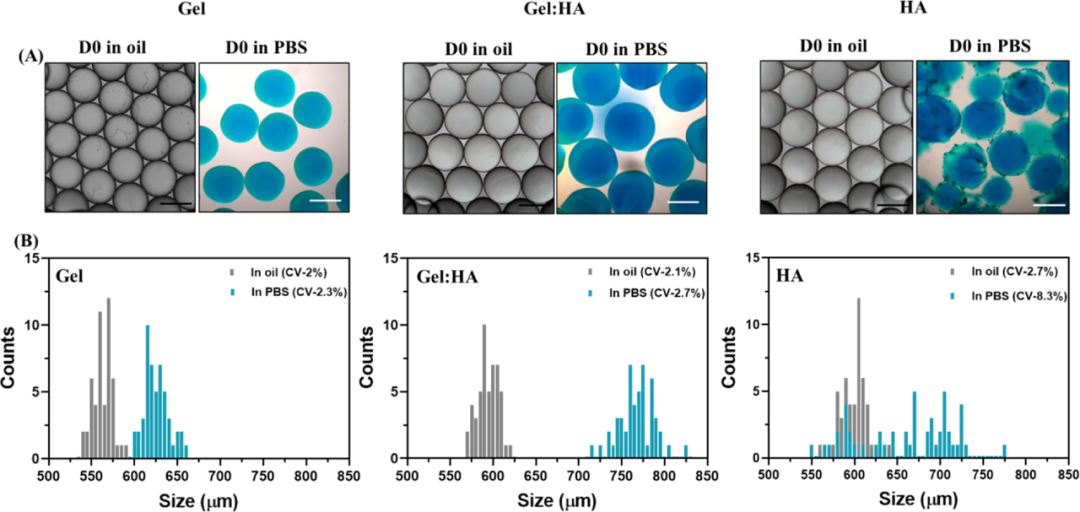
Next, the author uses pipette-based microfluidic technology to synthesize Gel, Gel:HA and HA microgels. The size and distribution of the microgels were characterized. Gel: HA microgels are consistent with the swelling observed in bulk hydrogels, both due to the high hydrophilicity of HA (Figure 3).
Figure 3 Characterization of Gel, Gel: HA, HA microgel
In order to understand the stability and suitability of hydrogels for cell culture, the authors measured the in vitro degradation rates of three hydrogel preparations in large volume and microgel state. Compared with block hydrogels, the quality of Gel:HA and HA hydrogels in DPBS is significantly increased. The gel quality of all volumes of hydrogels remained relatively stable within 10 days. After 10 days, a gradual increase in the quality of all hydrogel components was observed, with a higher proportion of HA increase. At the same time, the degradation performance of the microgel is significantly different from that of the overall hydrogel. The increase in the specific surface area of the microgel and the block hydrogel increases the mass transfer and causes the block hydrogel to degrade faster. This effect is more obvious for the highly hydrophilic HA microgel, which may be due to High degree of swelling (Figure 4).
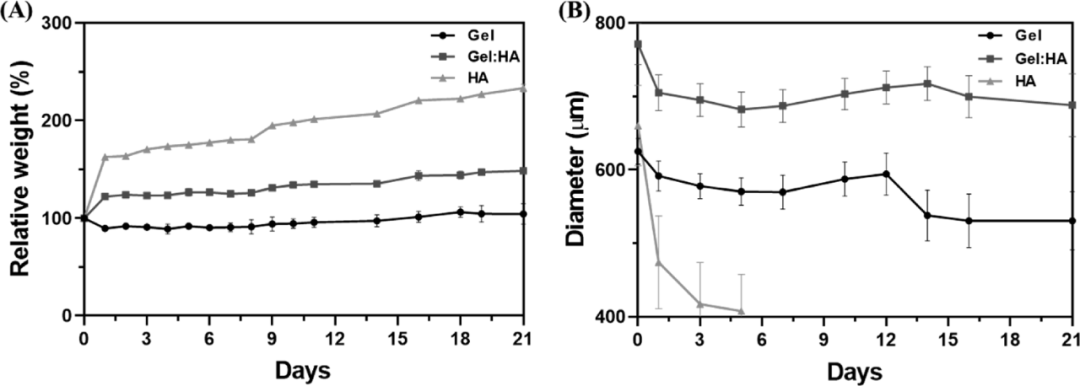
Figure 4 (A) Schematic diagram of the thiol-norbornene click reaction; (B) Schematic diagram of the preparation of bulk hydrogel and microgel
In order to evaluate the interaction of hydrogel composition and geometric structure on biological functions, the authors evaluated the ability of different hydrogel compositions to support hBMSC culture in block and microgel forms. The hBMSCs were encapsulated in the form of blocks and microgels, and after 1 day and 7 days of culture in the basic medium, the cell survival rate was evaluated by live/dead staining. After 24 hours of encapsulation and 7 days of culture, the cell survival rates of the three-component bulk hydrogel and microgel exceeded 85%. The HA hydrogel can be attributed to the lack of cell adhesion and diffusion in the hyaluronic acid hydrogel alone, which is necessary for anchoring dependent cells such as mesenchymal stem cells for survival and growth. These images also provide a preliminary indication of the cell distribution. Mesenchymal stem cells are distributed in the microgel on the first day in a circular shape, but migrate to the periphery of the microgel and diffuse before the seventh day, which makes the fluorescence more Strong (Figure 5).
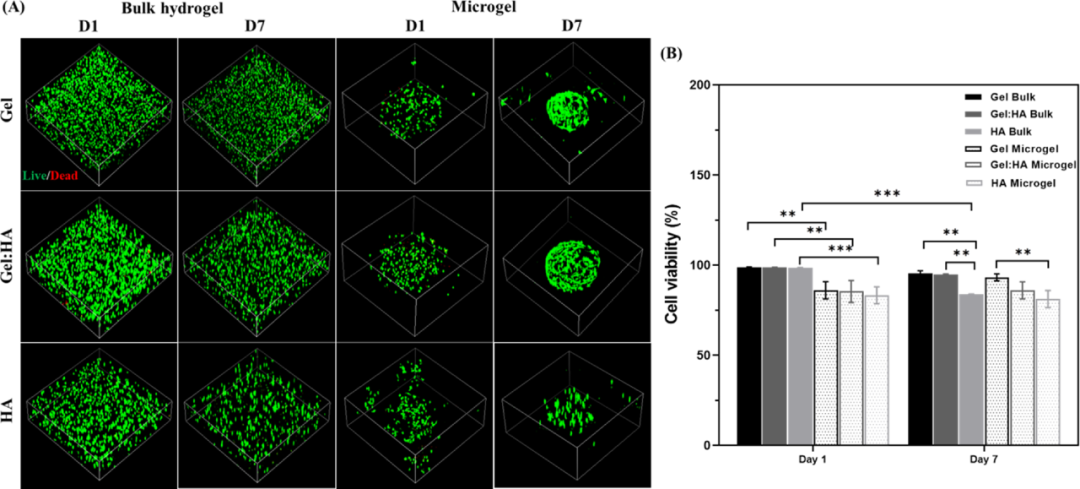
Figure 5 Evaluation of cell viability of hBMSCs encapsulated by block hydrogel and microgel
After that, the proliferation and cytoskeleton morphology of hBMSC were further analyzed. The results show that the cells in Gel and Gel:HA hydrogels are well distributed and prominent, indicating good interaction and adhesion with the polymer matrix. In contrast, the cells in the HA hydrogel remained round, possibly due to the lack of binding sites in HA. Regardless of the composition, the distribution of encapsulated cells is uniform for the bulk hydrogel. In contrast, almost all encapsulated cells in Gel and Gel:HA microgels migrate to the periphery of the microgel, but this effect is not obvious. The author speculates that this may be due to the cells actively sensing the higher mass transfer and nutrient concentration on the surface of the microgels, thereby migrating to the surface (Figure 6).
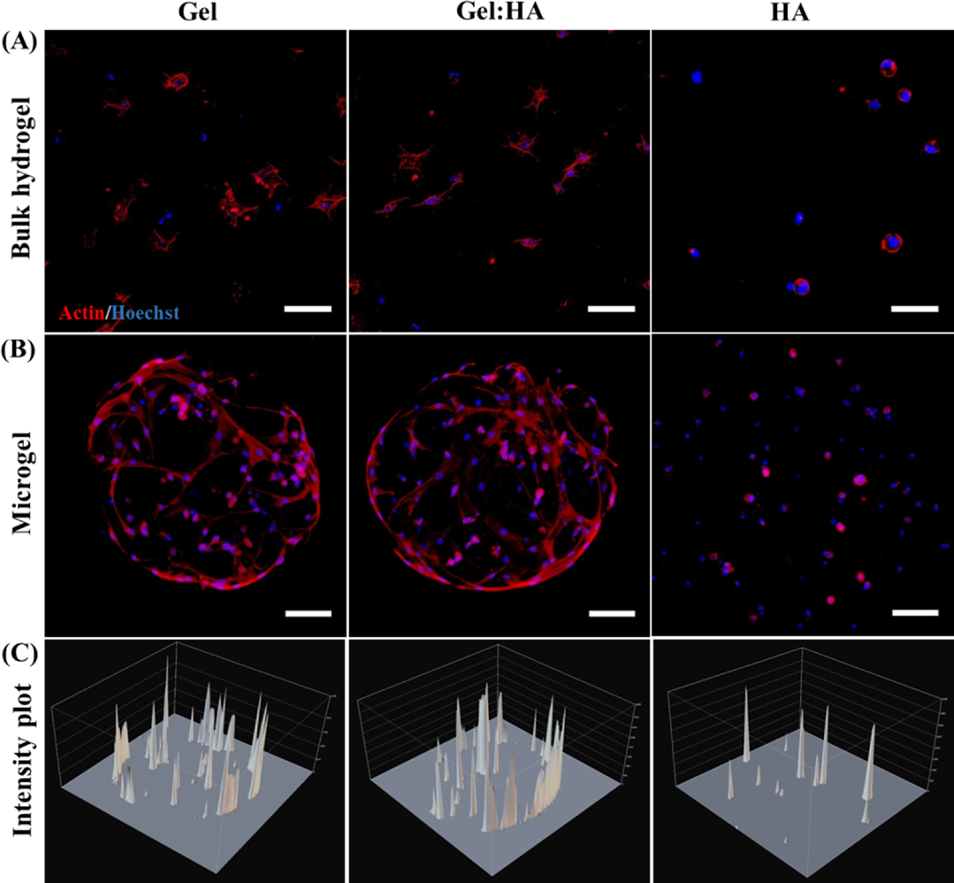
Figure 6 Cell morphology in block hydrogel and microgel
In order to determine the influence of the composition and geometry of the hydrogel on the differentiation of mesenchymal stem cells, all the volumes of hydrogels and microgels were cultured in basal medium and osteoinductive medium for 7 days. Compared with basic culture, the expression of ALP and RUNX2 genes were significantly up-regulated in osteoinductive culture, indicating that hBMSC successfully differentiated into osteoblast cell lines. In addition, the expression of ALP and RUNX2 in the osteogenic induction culture of hBMSC in Gel and Gel:HA was significantly higher than that of 2D monolayer cell culture. This emphasizes the advantages of the 3D platform over the 2D platform. In contrast, HA hydrogel does not support osteogenesis. Similarly, the expression of ALP in the microgel was significantly higher than that in the bulk gel (Figure 7).
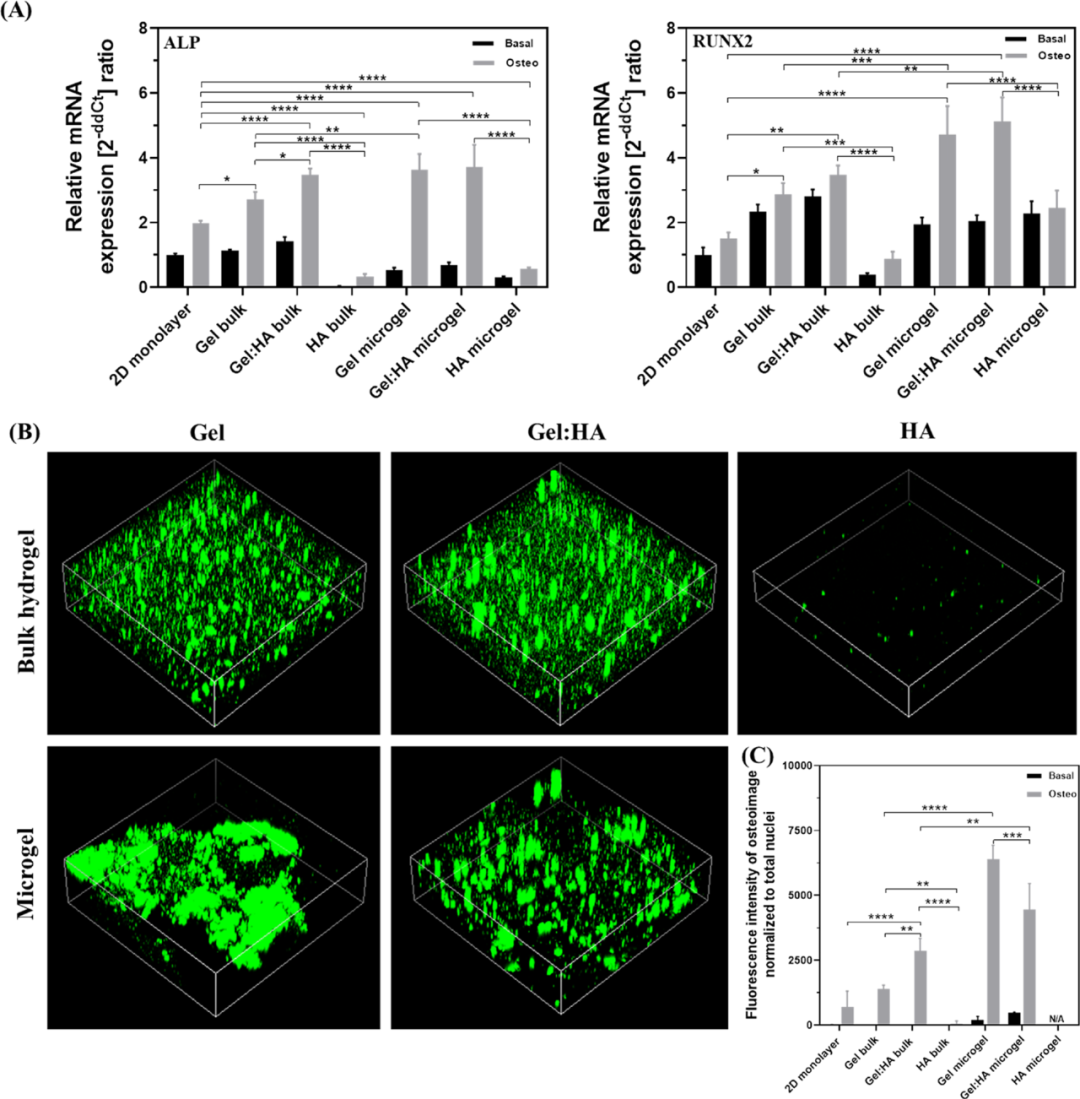
Figure 7 Osteogenic differentiation of hBMSCs in 2D, block hydrogel and microgel to detect osteogenic differentiation markers
Finally, in order to verify the hypothesis that the affinity of different hydroxyapatite to Gel and HA is the reason for the difference in mineralization, the authors tested whether the nucleation and retention of hydroxyapatite (HAP) are affected by different hydrogel compositions. Impact. By incubating hydroxyapatite nanoparticles (200 nM) with cell-free microgels for 24 hours, and then washing the excess hydroxyapatite nanoparticles, the effect of hydroxyapatite on each hydrogel used in the study was tested. Affinity. The bright-field image clearly shows the difference in the composition of hydroxyapatite deposits on the surface of the microgel. Gel microgel deposits the most, followed by Gel:HA and HA microgel. Hydroxyapatite is bound to the surface of the microgel. It is further illustrated by bone image staining and intensity quantification that Gel shows stronger fluorescence than Gel:HA and HA. As a source of collagen, gelatin may be beneficial to provide nucleation sites for the formation of apatite crystals. Studies on several glycosaminoglycans have shown that HA has the weakest affinity for hydroxyapatite (Figure 8).
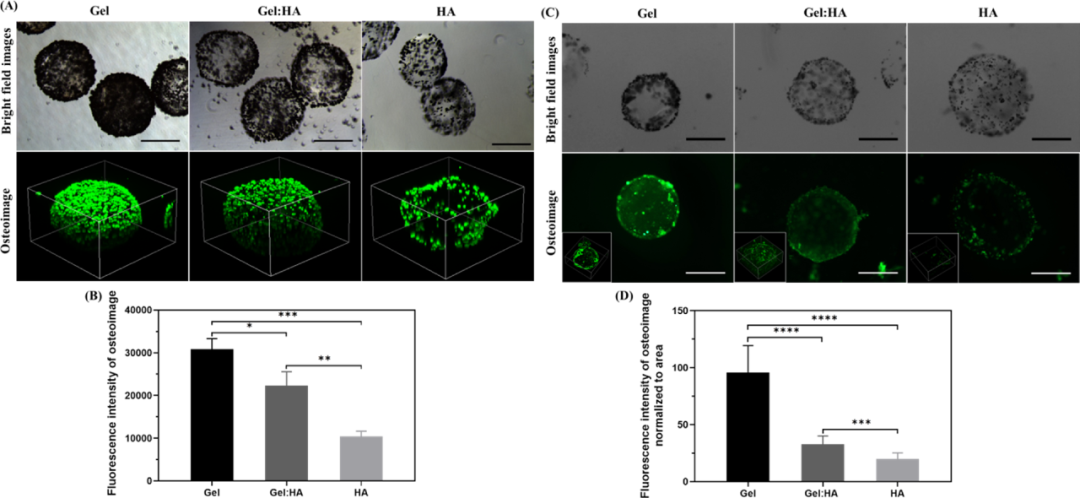
Figure 8 Hydroxyapatite affinity and nucleation research analysis. In this study, the author systematically studied the influence of hydrogel composition and geometric structure on hBMSC encapsulation and osteogenesis.for new composite materials for the design of hydrogels for tissue engineering purposes.
Information source: EngineeringForLife
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately












