
Chiral separation is a major challenge facing separation science. At present, chiral separation mainly relies on chromatographic column separation technology. However, the application of membrane technology in chiral separation is difficult and its development is relatively slow. Therefore, it is of great significance to develop a simple and rapid method for the synthesis of chiral nanoporous graphene membrane structures for the separation of highly selective enantiomers.
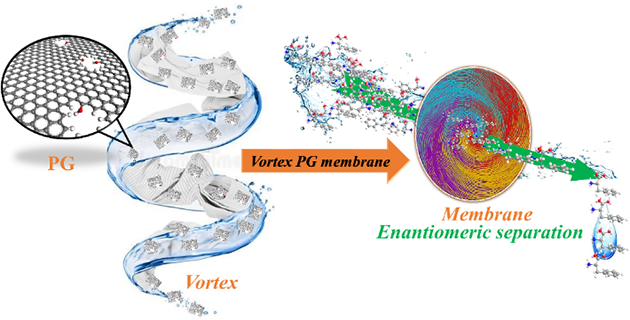
Vortex stacked porous graphene for chiral enantiomeric membrane separation
The Chiral Separation and Micro-Nano Analysis Group of Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences has long focused on the synthesis of nanoporous graphene and its application in separation analysis. Recently, the research team expanded the process of preparing graphene oxide by the Hummers method. The graphite oxide solution of the sulfate intercalation product in the high temperature stage of the Hummers method was directly suction filtered and dried to form two-dimensional nanoporous sulfuric acid between the layers of graphite oxide. Salt hydrotalcite template. During combustion, the exposed graphene in the pores of the hydrotalcite template is oxidized and removed. A new one-step synthesis method (patent application number: CN201811579832.8) directly from graphite to nanoporous graphene has been developed, which realizes the synthesis of nanoporous graphene. Simple, fast, efficient and low-cost one-step synthesis. The method uses graphite instead of graphene oxide, and at the same time uses the by-product of the Hummers method as a pore-making template, thereby eliminating the tedious and long water washing process in the synthesis of graphene oxide.
In the early stage, the research team has successfully prepared a single-layer nanoporous graphene ion separation membrane using a heavy ion accelerator (Analytical Chemistry, 2016, 88, 10002-10010), and subsequently developed a simple and rapid novel synthesis of nanoporous graphene by combustion Method (Advanced Functional Materials, 2018, 28, 1805026; Chinese Chemical Letters, 2019, 30, 863-866) and interlayer confined synthesis technology of metal oxide nano two-dimensional materials (Chem. Nano Mat., 2020, 6, 1270-1275, Wiley Chem platform special report), and successfully applied nanoporous graphene to high-selectivity membrane separation of ions. At the same time, composite materials of nanoporous graphene and metal oxides with nanoenzyme catalytic activity were prepared (Analytical Chemistry, 2019, 91, 5004-5010; Sensors and Actuators B: Chemical, 2019, 290, 15-22).
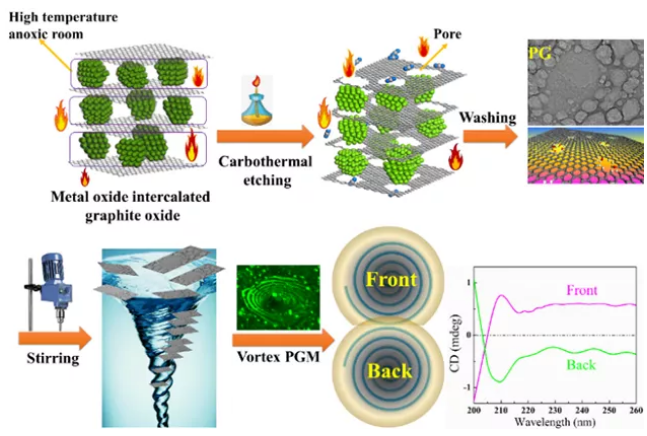
Synthesis and characterization of vortex porous graphene membrane
The research team members used a combination of mechanical stirring and rapid suction filtration to prepare nanoporous graphene with different pore sizes into nanoporous graphene separation membranes. The micron-scale vortex structure on the surface of the nanoporous graphene film was successfully observed using a laser confocal microscope, and it was confirmed that the vortex structure film has certain chiral characteristics, and the front and back sides of the film have opposite optical rotation characteristics. At the same time, the use of this chiral membrane successfully achieved the highly selective separation of racemic phenylalanine, with a maximum separation factor of 4.76. This work was recently published in Analytical Chemistry, 2020, 92, 13630–13633. PhD student Tan Hongxin is the first author of the paper, and associate researcher Li Zhan and researcher Qiu Hongdeng are the co-corresponding authors.
Source of information: Key Laboratory of Northwestern Featured Plant Resources Chemistry, Lanzhou Institute of Chemical Technology, Chinese Academy of Sciences
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately






