 The destruction or dysfunction of skin tissue integrity can significantly reduce the patients quality of life. Wound management, especially for chronic wounds (such as infected wounds, venous leg ulcers and diabetic foot ulcers), has become a basic medical and health care problem, which greatly increases the economic burden of the public. Skin wound recovery includes hemostasis, inflammation, proliferation and wound remodeling, and scar tissue formation. In order to accelerate this healing process, various biomaterials have been designed and manufactured to promote wound closure and healing. Injectable hydrogel has excellent wound exudate absorption performance, moisturizing ability and oxygen permeability, and has been widely accepted as the most attractive wound dressing. It has shape adaptability and can cover irregular wounds.
The destruction or dysfunction of skin tissue integrity can significantly reduce the patients quality of life. Wound management, especially for chronic wounds (such as infected wounds, venous leg ulcers and diabetic foot ulcers), has become a basic medical and health care problem, which greatly increases the economic burden of the public. Skin wound recovery includes hemostasis, inflammation, proliferation and wound remodeling, and scar tissue formation. In order to accelerate this healing process, various biomaterials have been designed and manufactured to promote wound closure and healing. Injectable hydrogel has excellent wound exudate absorption performance, moisturizing ability and oxygen permeability, and has been widely accepted as the most attractive wound dressing. It has shape adaptability and can cover irregular wounds.
The adhesion and retention of the wound site and the regeneration characteristics of biomaterials are important factors for skin tissue regeneration. Adhesive chemistry inspired by marine mussels reveals the development of functional adhesive hydrogels. Marine mussels secrete a catechol-containing binder solution at low pH (< 5). The solution contains weak mono-complexed iron (iron). The mono-complexed iron is further converted to di-or tri-iron in seawater. -Complexes to enhance underwater bonding. Protocatechualdehyde is a natural phenolic aldehyde with interesting molecular structure and can be used as a bonding motif through the formation of reversible covalent bonds including Schiff base bonds and catechol metal ion coordination chemistry. Polyamide has been proved to have pro-apoptosis and antibacterial properties, and has been used in clinical applications to treat coronary heart disease, indicating that polyamide has good biocompatibility. At the same time, the catechol group-containing material exhibits good biocompatibility and free radical scavenging ability, as well as the photothermal ability after complexing with iron to inactivate bacteria, which is required for wound healing. In addition, hydrogels cross-linked through dynamic bonds generally exhibit autonomous healing capabilities and stimulus response characteristics, which help control the removal of dressings and avoid trauma to epithelialized tissues.
Infection caused by bacterial contamination is still one of the obstacles to wound healing. To solve this problem, various antibacterial materials have been integrated into hydrogels, including antibacterial hydrogels based on aminoglycosides. Although the antibacterial effect is strong, the development of drug resistance due to the improper use of antibiotics cannot be ignored. Therefore, there is an urgent need to design a hydrogel with inherent antibacterial properties, which can effectively inactivate bacteria without drug intervention. Minimal invasive photothermal therapy (PTT) has been generally accepted as a promising alternative to antibiotic treatment against infections, especially for resistant bacteria. The most commonly used photothermal agents are composed of carbon-based composite materials, precious metal nanomaterials, and metal sulfide nanomaterials. However, the controlled synthesis, removal and potential toxicity of nanoparticles are still challenges to be overcome. Therefore, the development of nanomaterial-free hydrogels with photothermal properties as wound dressings is still highly anticipated.
Based on this, Guo Baolin and others from Xian Jiaotong University designed a hydrogel with good performance through the dual dynamic cross-linking reaction of polyamide containing iron, catechol and aldehyde groups with QCS. The use of pH-sensitive coordination bonds (iron catechol) and dynamic Schiff base bond double cross-linking, as well as reversible rupture and reformation, improve the mechanical properties of the hydrogel, and give the hydrogel injectable Sex and self-healing properties. At the same time, the catechol iron cross-linked matrix shows good photothermal ability. Combined with the inherent antibacterial activity of QCS, hydrogels show good application prospects as a substitute for antibiotics. In addition, catechol and aldehyde groups used for dynamic crosslinking impart excellent adhesion to the hydrogel. More importantly, the pH sensitivity and stability of the catechol iron bond depend on the addition of iron chelating agents, which can dissolve or remove viscous hydrogels as needed after appropriate stimulation. This work is titled "Dual -Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing was recently published on ACS Nano , proving that it can handle the full-thickness of skin incisions and infections Great potential in skin wounds.

Preparation of hydrogel
In order to design a double dynamic bond cross-linked self-healing hydrogel with biocompatibility, excellent antibacterial ability and good adhesion, QCS and a stable three-complex molecule iron (PA) 3 (PA) were first synthesized. @Fe) (Figure 1). QCS was synthesized by grafting glycidyl trimethyl ammonium chloride (GTMAC) on the chitosan chain. The double dynamic bond cross-linked hydrogel is prepared by mixing QCS and polyamide @铁 by Schiff base bond (Figure 2A). The coordination of iron and polyamide can be used as a bonding motif between QCS molecules to improve QCS. Mechanical properties of hydrogels. As the molar ratio of amino groups to aldehyde groups increased from 40:1 to 8:1, the maximum compressive stress of the hydrogel increased from 2.2 N to 15.0 N (Figure 2C).

Figure 1 Schematic diagram of preparation and application of viscous hydrogel
Hydrogel material performance characterization
The gel time is important for the application of hydrogels as biomaterials. The storage modulus (G) and loss modulus (G) of the gel were tested on a rheometer with time (Figure 2D), and the gel time was determined as the intersection of G and G.55. With the increase of iron phosphate content, the gel time decreased from 50 minutes to 10 minutes, and the Gvalue increased from 270 Pa to 1980 Pa, indicating that the double dynamic bond cross-linking interaction can improve the mechanical properties of the gel. In addition, it is generally believed that hydrogel as a dressing can absorb tissue exudates, maintain a moist environment, and facilitate wound recovery. Therefore, the swelling rate of the hydrogel was tested in PBS (pH 7.4) at 37°C. In Figure 2E, the swelling rate of the wet hydrogel decreased from 267% to 174%. After soaking in PBS for 12 hours, the molar ratio of amino groups and aldehyde groups changed from 20:1 to 8:1, which was attributed to coagulation. The crosslink density of the glue increases. QCS-PA@Fe40, which has the lowest crosslink density, shows lower superparamagnetism than other hydrogel samples. This may be due to the loose network structure of QCS-PA@Fe40, which is more likely to be corroded. The water condensation was observed by scanning electron microscopy. The morphology of the glue. This indicates that the prepared hydrogel has a porous structure (Figure 2G)

Figure 2 Characterization of hydrogel materials
Self-healing and injectability
Due to the shear thinning ability, hydrogels with excellent self-healing properties and shape adaptability characteristics have shown promising applications in wound closure. The viscosity of the hydrogel (QCS-PA@Fe10) decreases with the increase of the shear rate (Figure 3A), which proves the shear thinning ability of the gel. At the same time, the hydrogel can be continuously injected through a syringe to draw the letters of "XJTU" and maintain a stable gel state when the shear force is removed.

Figure 3 Injectability and self-healing properties of hydrogel
Biocompatibility
Since the dissolution of chitosan occurs under the intervention of acid or urea/alkali, QCS was selected as the control group for in vivo biocompatibility evaluation to avoid the influence of additional materials on the biocompatibility evaluation. At the same time, QCS has good biocompatibility. After the hydrogel samples were embedded subcutaneously for 1 week, all groups had acute inflammation. As the hydrogel degraded, inflammatory cells and inflammatory reactions gradually decreased. The mild inflammatory response of the sample after 4 weeks of implantation proved that the designed hydrogel has good compatibility in vivo. These results indicate that the antioxidant hydrogel has good biocompatibility and shows good application prospects in wound healing.

Figure 4 Biocompatibility of hydrogel
Antibacterial properties
The antibacterial properties of the gel against Gram-negative Escherichia coli, Gram-positive Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus were evaluated by the surface antibacterial activity test (Figure 5A-F). After being in contact with the hydrogel at 37°C for two hours, most of E. coli (>90%) and Staphylococcus aureus (~80%) were inactivated by these hydrogels, which proved the resistance to E. coli and Staphylococcus aureus The excellent antibacterial properties.
Near-infrared auxiliary antibacterial activity
After photothermal treatment, the mice were sacrificed, and the healed skin was collected and homogenized to evaluate the antibacterial effect of the gel. The number of surviving colonies was determined by standard plate counting method. As shown in Figure 5K, the blank group with or without near-infrared radiation intervention, and the hydrogel-treated group without near-infrared radiation intervention, did not show a significant reduction in bacteria. However, compared with the three control groups, the number of bacteria in the group treated with the near-infrared radiation hydrogel was significantly reduced (P <0.05) (Figure 5L). The above results indicate that the antibacterial hydrogel can be used as a wound dressing to promote wound healing of infected skin.

Figure 5 Antibacterial properties of hydrogel
Adhesion, removal and hemostatic ability
Through the lap shear test with pig skin as a model biological tissue (Figure 6A). As the iron phosphate content increased, the hydrogel showed an increased bond strength ranging from 3 to 40 kPa (Figure 6B). After external force is involved, the sticky pig skin is broken, and then the cut skin of the broken hydrogel is put together, and the reopened cut can be glued with viscous hydrogel (Figure 6C, D). Record the amount of blood lost during hemostasis after the application of the hydrogel or without treatment. In the two bleeding models, the hydrogel-treated group showed better hemostatic performance because almost no obvious bloodstains were detected (Figure 6H), while the untreated group showed significant bleeding.

Figure 6 Adhesion performance of QCS-PA@Fe10
Wound closure and healing in the body
A full-thickness skin incision model was used to evaluate the wound closure performance of QCS-PA@Fe10 hydrogel. A skin incision (2 cm, full thickness) was made on the back of the rat; then the incision wound was treated with surgical sutures, biomedical glue and viscous hydrogel, and the untreated wound was set as a control (Figure 7A). The incision treated with hydrogel shows better closure than surgical sutures and biomedical glue. At the beginning of the closure, the untreated incision became larger due to the movement of the rat, while the hydrogel-treated incision remained well closed.
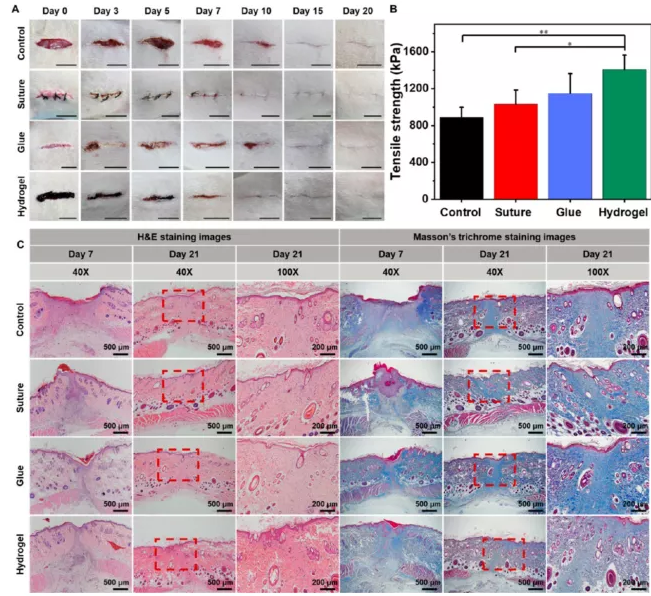
Figure 7 Wound healing evaluation of hydrogel
Wound healing of methicillin-resistant Staphylococcus aureus infection in vivo
As shown in Figure 8A-C, after 3 days of healing, wounds treated with Tegaderm dressing, pure QCS and hydrogel showed obvious pathogen contamination, while wounds treated with hydrogel showed almost no contamination with near-infrared radiation. The regenerated skin tissue treated with the near-infrared radiation hydrogel showed more blood vessels and hair follicles. All these results indicate that the double cross-linked adhesive hydrogel is an excellent candidate for antibacterial wound dressings. At the same time, collagen deposition was evaluated by Masson trichrome staining (Figure 9A). Blue means collagen, red means keratin or muscle fibers. The results showed that after 1 week of healing, the skin tissue showed obvious collagen deposition, and the hydrogel group irradiated by near infrared showed the highest collagen deposition.

Figure 8 Evaluation of antibacterial performance

Figure 9 Biochemical characterization
to sum up
The double dynamic bond cross-linked network gives the hydrogel good mechanical strength and tissue adhesion, as well as excellent self-healing ability. In addition, the inherent antibacterial properties of QCS and near-infrared assisted photothermal ablation synergistically enhance the antibacterial efficiency of the hydrogel. All these desirable properties give the hydrogel effective wound closure and significantly promote wound healing. In addition to effectively sealing the skin incision, the double dynamic bond cross-linked hydrogel can be removed on demand under the intervention of DFO or acid solution. With all the desired properties mentioned above, the dual dynamic binding cross-linked viscous hydrogel can effectively seal skin incisions and perform post-wound closure care, which is shown in the healing of full-thickness skin wounds with methicillin-resistant Staphylococcus aureus infection The positive effect shows its great potential as a wound dressing.














