
[abstract].
Platelets infiltrate into the tumor microenvironment from circulation, promote metastasis, and give several cancers resistance to chemotherapy. Therefore, using effective and non-toxic antiplatelet drugs combined with anticancer drugs to prevent tumor-platelet crosstalk may be an effective cancer treatment strategy. To test this concept, Professor Abhishek Jain at Texas A & M University created an ovarian tumor microenvironment chip (OTME-Chip), which consists of a tumor microenvironment with platelet perfusion, which outlines platelet extravasation and its consequences.
Through tumor and RNA sequencing including gene editing, the organs on the chip reveal that platelets and tumors interact with tumor galactose lectin-3 through the glycoprotein VI (GPVI) under shear. Finally, as proof of the principle of clinical trials, the team showed that the GPVI inhibitor Revacept impaired metastatic potential and improved chemotherapy. Because GPVI is an antithrombotic target that does not damage hemostasis, it represents a safe cancer treatment. The team suggested that OTME-Chip could be deployed to study other vascular and hematological targets in cancer. The related paper is published in Science Advance under the title Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer.
[picture and text analysis].
OTME-Chip s formation team was inspired to design an organ chip microfluidic platform that acts as a 3D organ simulation in vitro model of the tumor microenvironment, connected to vascular and platelet flow (figure 1A). It retains some elements of their recently released OvCa-Chip existing platform, a dual-lumen organ chip technology consisting of cancer cells co-cultured with 3D endothelial vessels and separated by matrix-coated polydimethylsiloxane (PDMS) membranes. For details, see Materials and methods)-but in order to conduct a longitudinal study of cancer progression and analyze the effects of extravasated platelets on cancer cell proliferation and invasiveness, we completely redesigned the top tumor chamber of the device by adding two adjacent ECM chambers, consisting of a side PDMS microcolumn array (figure 1B).
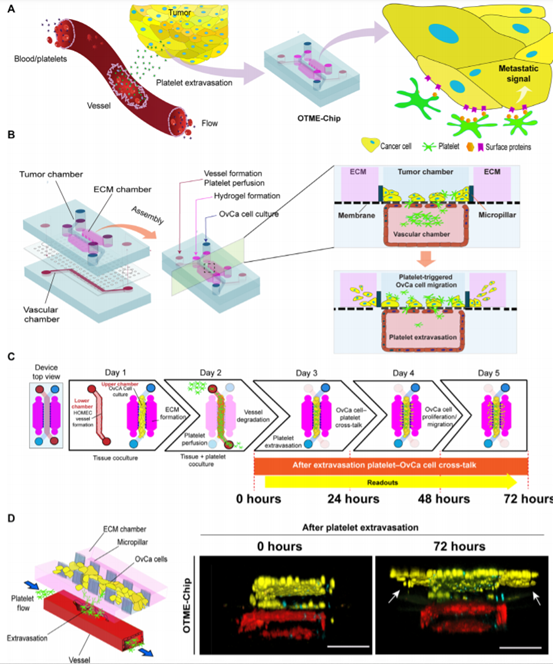
Figure 1.
Micro-engineering of OTME-Chip.
This biological system design strategy, now known as OTME-Chip, is inspired by different organ chip technologies that simulate the metastatic invasiveness of colorectal and breast cancer cells using artificially created ECM simulation materials. This design does not allow blood perfusion or close analysis of the interaction between cancer cells, vascular cells and blood components. However, in OTME-Chip, the integration of OvCa-Chip with this pillar structure design provides the team with the first opportunity to study critical platelet-cancer cell interactions under flow, while still keeping the technique relatively simple and easy to adapt. First, the team established an ECM compartment by injecting a pre-gel solution made from type I collagen, and incubated the chip to form a semi-solid hydrogel limited by microcolumns. Next, human ovarian microvascular endothelial cells (HOMECs) were inoculated into the lower chamber of the device to form a complete 3D vascular lumen.
Platelets interact with cancer cells through the binding of GPVI and galectin-3 under shear. In several cancers, including ovarian cancer, the expression of galactose lectin is up-regulated and induces platelet adhesion and hyperactivity. Adhered and activated platelets in turn prepare for cancer cell metastasis. Tumor galactose lectin-3 specifically contains a collagen-like domain. On the other hand, the collagen receptor GPVI on platelets regulates platelet activation under shear. Therefore, the team examined whether OTME-Chip could reveal the contribution of GPVI-galectin-3 binding platelet-cancer cell interaction and its metastatic consequences under shear (figure 2A). The team first examined the expression and activity of galectin-3 during the 72-hour timeline of cancer cells exposed to exosmosis platelets and found that galectin-3 expression was conservative and did not change with time (figure 2B).
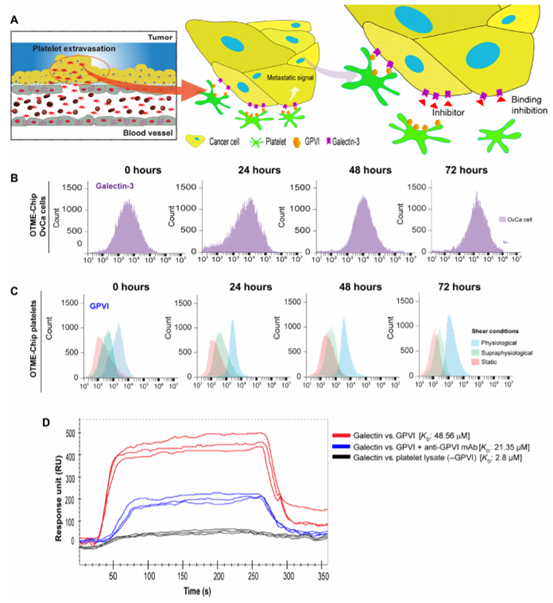
Figure 2.
The expression of platelet GPVI is cut-dependent and binds to tumor galectin-3 in OTME-Chip.
OTME-Chip was used to evaluate the consequences of the interaction between GPVI and galectin-3 in ovarian cancer, and then the possibility of GPVI-galectin-3 interaction as a mediator of platelet-promoted ovarian cancer metastasis was studied. To test this hypothesis, the team used CRISPR-Cas9 editing to establish an ovarian cancer cell line with galectin 3 knockout (KO-g3) (figure 3A). The parameters (ECM invasion, proliferation, cytokine profile and transcriptional reading) controlling cancer cell metastasis in OTME-Chip composed of primitive ovarian cancer cells (OTME-Chip) or galectin 3 knockout cancer cells (KO) were compared. It was found that when co-cultured with exosmosis platelets (OTME-Chip) versus control without platelets (Control-Chip), wild-type ovarian cancer cells quickly invaded hydrogel ECM. However, a decrease in ECM invasion in KO-OTME-Chips was observed in the presence of exosmosis platelets (figs. 3, B and C).Within 40 to 72 hours of the interaction between platelet extravasation and tumor, cancer cell proliferation increased in OTME-Chips compared with the control, and decreased proliferation was found in galectin-3 KO-OTME-Chips (figure 3D). The proliferation of OvCa cells was observed in direct proportion to the invasion of ECM hydrogel in the observed timeline.
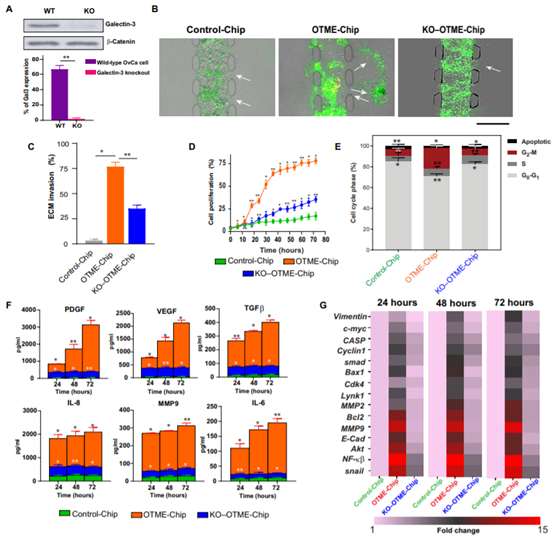
Figure 3.
Platelet GPVI promotes ovarian metastasis through galectin-3 that binds to cancer cells in OTME-Chip.
OTME-Chip predicted that drug inhibition of GPVI could prevent metastasis and supported the chemotherapy team found that OTME-Chips (Rx-OTME-Chips) treated with Revacept showed a significantly reduced GPVI-galectin-3 binding kinetics (figure 4A). Revacept significantly reduced the ECM invasion and proliferation rate of cancer cells indicating that GPVI inhibition also prevented the consequences of GPVI- galectin-3 interaction (Fig. 4).
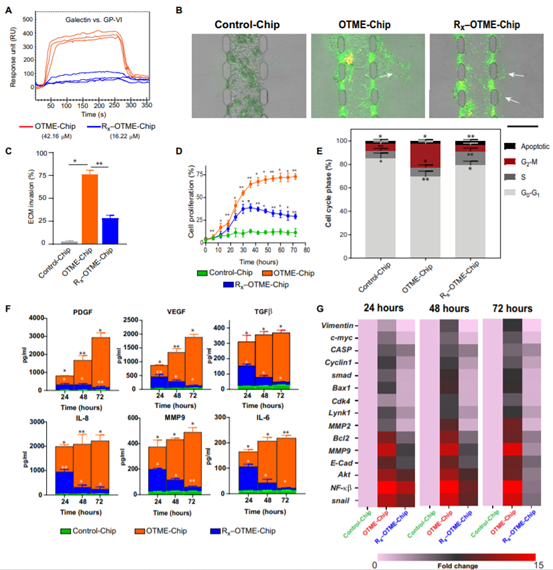
Figure 4.
Revacept (GPVI inhibitor) prevents ovarian metastasis in OTME-Chip.
In addition, since platelets have been shown to promote chemical resistance to tumors, the team examined the effect of GPVI-galectin-3 interaction and blocked the chemical resistance to ovarian cancer cells by Revacept. Our OTME- chip was treated with no drug (OTME-Chip) or cisplatin (Cx-OTME-Chip), an anticancer drug close to the clinically relevant concentration (6 ¦Ì g/ml), or cisplatin (CxRx-OTME-Chip) combined with Revacept. We found that cisplatin alone had a moderate effect, while cisplatin-Revacept combination had significant effects on growth factors and cytokines in reducing tumor invasiveness (figure 5A), cell proliferation (figure 5B), G2murM phase (figure 5C) and concentration (figure 5D). Finally, transcriptional analysis of cancer cells obtained from the chip showed that when OTME-Chip (Cx-OTME-Chip) contained cisplatin alone, platelets initiated the upregulation of metastasis and cell proliferation genes with moderate changes over time; however, Revacept significantly reduced this overexpression (CxRx-OTME-Chip; figure 5e).
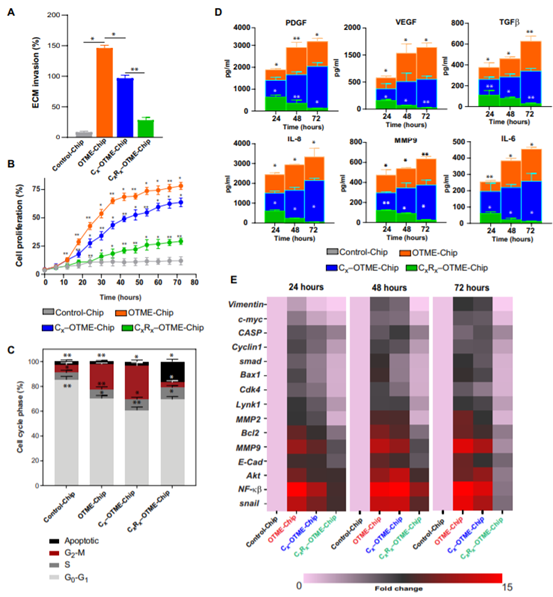
Figure 5.
Revacept (GPVI inhibitor) reduced the chemical resistance in OTME-Chip.
Using next-generation RNA-seq to verify cancer cell-platelet-drug crosstalk organ chip technology in OTME-Chip and RNA-seq analysis is a powerful tool for verifying preclinical research and obtaining fair clinical predictions. Therefore, in order to consolidate the teams views on the platelet-tumor signal transduction relationship and the therapeutic efficacy of Revacept, transcriptome analysis of tumors derived from different chips was performed by RNA-seq (figure 6A). Through RNA-seq and differential gene analysis, 12452 genes were detected in OTME-Chip relative to Control-Chip expression. Similarly, the team detected the expression of 5566, 6909 and 3821 genes in Cx-OTME-Chip, CxRx-OTME-Chip and KO-OTME-Chip, respectively, compared to Control-Chip (figure 6B).
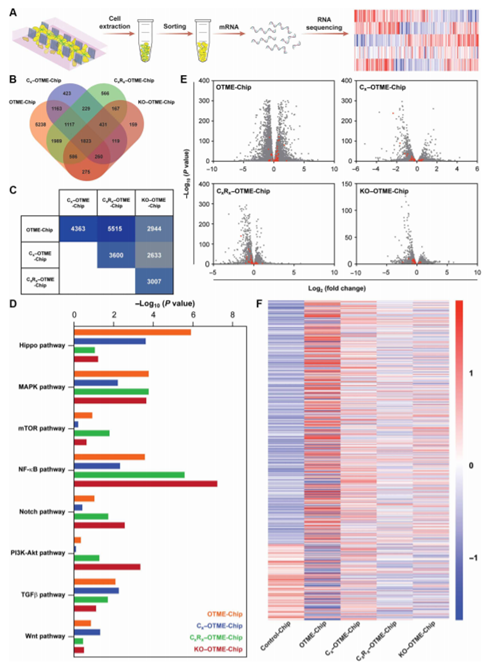
Figure 6.
RNA-seq and differential gene expression analysis revealed the efficacy of antiplatelet therapy in weakening tumor promotion pathway.
[summary].
The team demonstrated that the complex interaction between tumor and platelets and the resulting metastatic process can be modeled in a bioengineered tumor microenvironment with vascular components on the chip. The team found that the platform could be used to identify complex tumor-blood cell interactions, metastasis signals, and to evaluate drugs by integrating genomic and proteomic methods. In addition, the scope of application of the required anticancer or antiplatelet drugs alone or in combination makes our equipment a potential platform for drug evaluation of platelet-induced cancer metastasis and chemical resistance. The opportunity to include ECM in the chip also increases the additional ability to study tissue invasiveness and platelet-induced tumor invasion under different conditions. We expect that this OTME-Chip technology can further advance personalized cancer medicine by integrating induced pluripotent stem cells derived from malignant tumors and blood or immune cells from other patients, where we can analyze the interaction signals of potential metastasis.In summary, the human OTME-Chip model may provide a potential platform for studying the interaction between blood cells and cancer cells, obtaining functional readings and preclinical data of different antimetastatic drugs, and then start large animal studies and human clinical trials.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.










