已传文件:photo/1631586161.png

summary:
The highly complex tumor immunosuppressive microenvironment (TIME) composed of tumor cells, stromal cells and infiltrating immune cells is an important reason for the low efficiency of tumor chemotherapy and immunotherapy. Tumor-associated macrophages (TAMs) are one of the most abundant tumor-infiltrating white blood cells, especially M2 type TAMs play an important role in promoting tumor growth, angiogenesis, tumor metastasis and immune escape. Therefore, clearing or polarizing M2 tumor-associated macrophages is expected to reshape the tumor immunosuppressive microenvironment to improve the effectiveness of tumor treatment. However, due to the wide distribution of macrophages in the body and the key role in innate immunity, biological safety is the main obstacle to TAMs-based treatment. Therefore, how to achieve specific targeted therapy of M2 TAMs has always been a bottleneck problem in research.
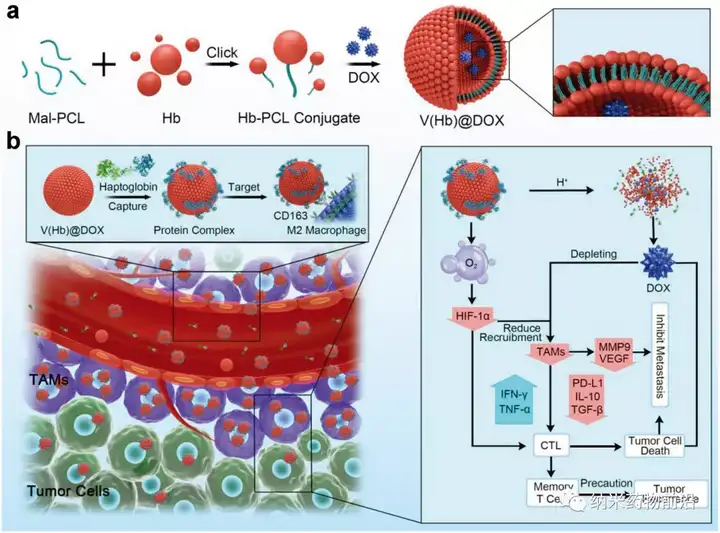
Recently, the team of Professor Zhou Dongfang from School of Pharmacy of Southern Medical University and Professor Huang Yubin of Northeast Normal University published an online publication titled "EngineeringEndogenous Tumor-Associated Macrophage-" in the internationally renowned academic journal Advanced Materials (DOI: 10.1002/adma.202103497) (IF: 30.849). Targeted Biomimetic Nano-RBC toReprogram Tumor Immunosuppressive Microenvironment for EnhancedChemo-Immunotherapy" research paper, and was selected as the cover article (InsideFront Cover). The research designed and synthesized a hemoglobin poly-coupled self-assembled biomimetic nano red blood cell system (V(Hb)) to deliver the chemotherapy drug doxorubicin (DOX) and oxygen to reprogram the tumor immune microenvironment. Utilizing the characteristic that hemoglobin can spontaneously bind to endogenous plasma haptoglobin (Hp) and specifically target M2 type macrophages through the CD163 surface receptor, the drug delivery system V(Hb)@DOX can achieve M2 type The endogenous specific targeted killing of TAMs, and the oxygen-carrying function of hemoglobin can improve the hypoxic microenvironment of tumors, thereby reshaping TIME and realizing efficient cancer chemotherapy-immunotherapy combined therapy. In orthotopic mouse breast cancer (4T1) metastasis model or subcutaneous mouse colon cancer (CT26) postoperative recurrence model, V(Hb)@DOX can significantly reduce TAMs and related immunosuppressive factors in tumors, and increase T cells Infiltration, effectively inhibits tumor metastasis and recurrence, and greatly reduces the related toxicity of small molecule drugs.
This research provides a biomimetic nano red blood cell delivery system that specifically targets TAMs, which can safely and efficiently target chemotherapeutics and oxygen, reshape TIME synergistically, and has broad application prospects in targeted immunotherapy of cancer TAMs. Professor Zhou Dongfang is the last corresponding author of this article, Professor Huang Yubin of Northeast Normal University is the co-corresponding author, and Wang Yupeng, a postdoctoral fellow at Southern Medical University, is the first author. The paper was supported by the National Natural Science Foundation of China and other projects.
Highlights:
1. Construct a hemoglobin-based biomimetic nano-red blood cell drug delivery system, and use the metabolic properties of hemoglobin to achieve endogenous and specific targeting of M2 type TAMs.
2. Endogenous specific targeted killing combined with oxygen supply synergistically reduce the content of TAMs in the tumor and reshape TIME.
3. Reshape TIME to achieve a safe and efficient cancer chemotherapy-immunotherapy combined treatment, which effectively inhibits tumor growth, metastasis and recurrence.
Schematic diagram:
Figure 1. A schematic diagram of engineered endogenous TAMs targeting biomimetic nano red blood cells to reprogram the tumor immune microenvironment to enhance cancer chemotherapy and immunotherapy. a) Schematic design of bionic nano red blood cell drug delivery system (V(Hb)@DOX). b) A schematic diagram of endogenous specific TAMs targeting V(Hb)@DOX through reprogramming TIME to enhance chemotherapy and immunotherapy.
Full text link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202103497
Cover link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202170304
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately