已传文件:photo/1766544328.png
In the current era of vigorous development in life sciences and precision medicine, how to clearly observe the fine structures of subcellular components under conditions without background interference and without light damage has always been the core challenge of imaging technology. Electrochemical Luminescence (ECL) technology is favored in the fields of biological sensing and imaging due to its advantages such as no need for external excitation light sources, nearly zero background, and strong temporal and spatial controllability. However, to apply it to high-resolution microscopic imaging, especially for observing ultra-fine subcellular structures, there are two major bottlenecks: (1) Weak luminescence signal: The existing ECL probes (including gold nanoclusters) have low quantum yield and weak signals, making it difficult to meet imaging requirements; (2) Inaccurate positioning: The probes are difficult to fix or the probe size is too large, severely limiting the spatial resolution. The ideal solution is to construct an ECL probe with a size smaller than 2 nm and be able to be anchored in situ and closely at the electrode-cell interface, forming a "near-zero thickness" luminescent source.
Recently, the team led by Zhu Junjie and Chen Zixuan from the School of Chemistry and Chemical Engineering of Nanjing University published a groundbreaking achievement in the journal Angew. Chem. Int. Ed. They successfully synthesized a class of gold nanocluster probes with extremely high ECL brightness. This probe achieves configurational rigidity through an intramolecular hydrogen bond network, not only increasing the ECL intensity by more than 250 times, but also constructing a "near-zero thickness" ECL luminescent layer with a thickness approaching the scale of a single cluster (~2 nm). This innovation enables researchers to clearly image the subcellular structure of cells with a spatial resolution of approximately 170 nm in a completely dark and background-free environment - equivalent to illuminating the cell with an "atomic-level LED lamp". This work not only solves the long-standing problem of low brightness and poor positioning of traditional ECL probes, but also provides a new paradigm for the next generation of high-resolution, non-labeled, in situ biological imaging, and is expected to play an important role in single-cell dynamic monitoring, organelle interaction research, and in situ imaging of disease markers. 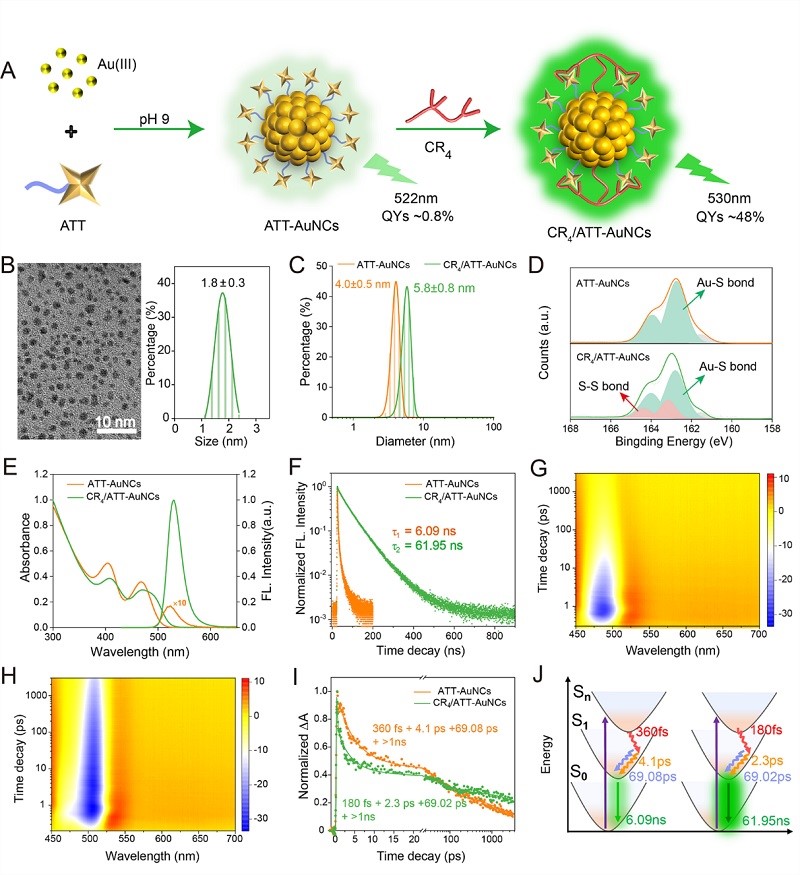
Figure 1. Hydrogen bond lock configuration, enhancing FL
The research team developed a gold nanocluster (CR4/ATT-AuNCs) protected by a combination of polyarginine peptide (CR4) and heterocyclic ligand 6-azido-2-thiothymine (ATT). The key innovation lies in the formation of a dense intramolecular hydrogen bond network between CR4 and ATT, which effectively rigidifies the ligand structure and significantly inhibits non-radiative transitions. This "hydrogen bond lock" strategy has increased the fluorescence quantum efficiency of the gold clusters by approximately 60 times. 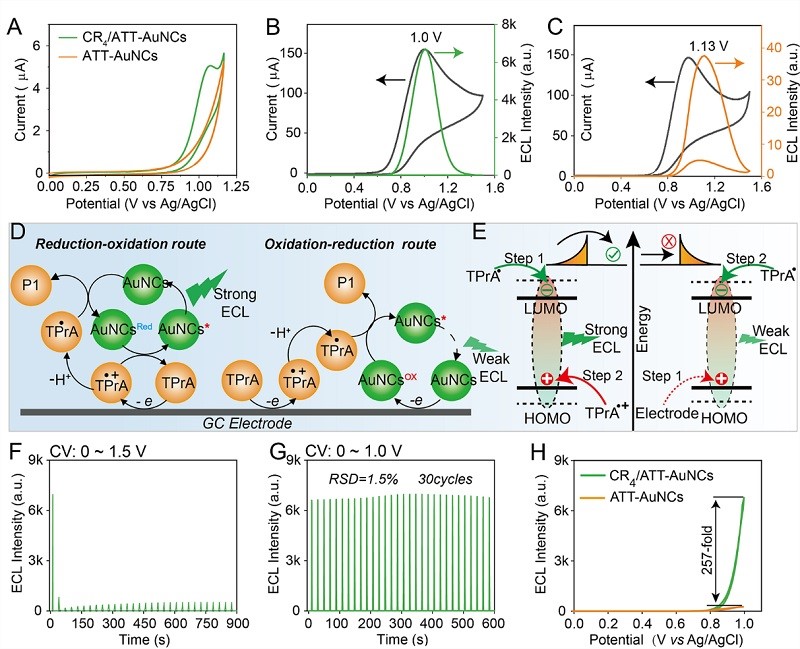
Figure 2. Hydrogen bond lock configuration, activating ultra-strong ECL
This strategy rigidifies the ligand conformation, and hydrogen bonds "lock" the ligand flexibility, significantly suppressing non-radiative transitions. This results in a 60-fold increase in the fluorescence quantum efficiency of the gold clusters, while promoting the π-π ordered stacking of ligands on the gold cluster surface, enhancing the electron transfer efficiency between the co-reactant (TPrA) and the gold clusters, and unexpectedly activating an efficient reduction-oxidation (Reduction-oxidation) pathway, causing the ECL intensity to increase by more than 250 times. 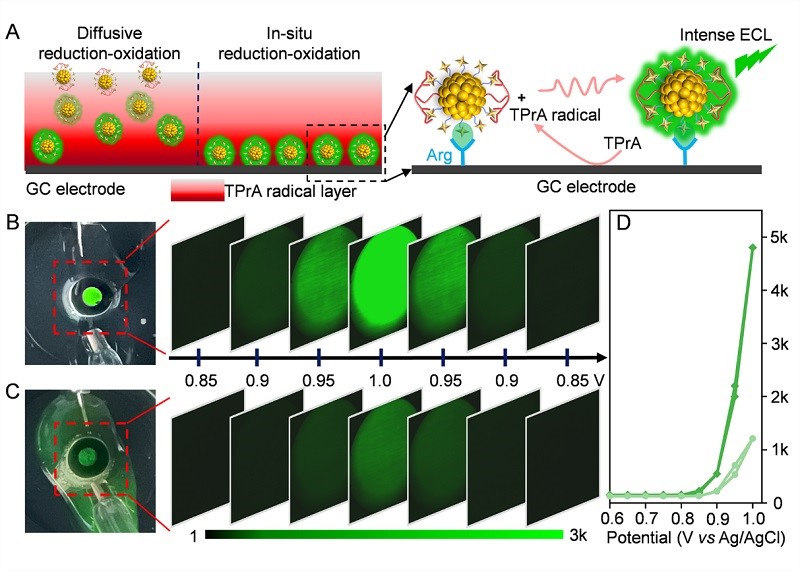
Figure 3. The visible single-cluster thickness luminescent layer
When CR4/ATT-AuNCs are fixed on the electrode surface, they can emit a bright green light that is visible to the naked eye when a voltage is applied. Through experimental characterization, the ECL signal originates from an interface layer with a thickness of only 2-3 nanometers - almost equivalent to the height of a single cluster. This truly achieves a "near-zero thickness" super-strong light source. This extremely thin layer significantly compresses the spatial dispersion of light emission, laying a physical foundation for high-resolution imaging. 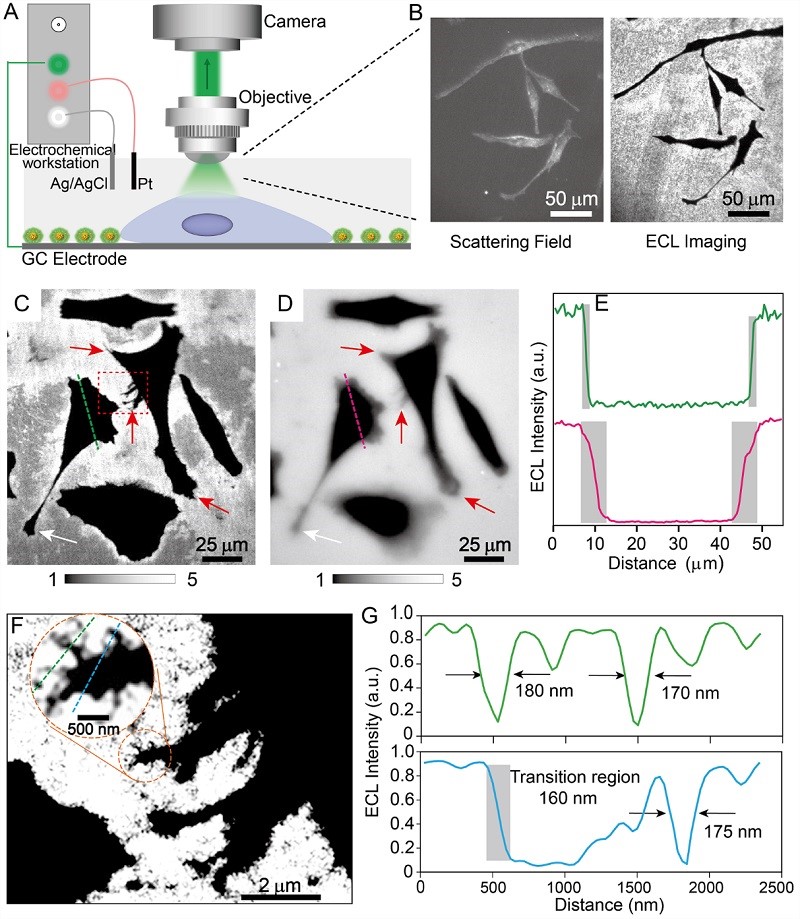
Figure 4. Super-resolution imaging of subcellular structure ECL
Using this highly luminous probe, the team built an ECL microscopic imaging system to perform in situ imaging of adherent HeLa cells. Without any additional excitation light source, the cell edges, pseudopod extensions, and local complex textures could be clearly distinguished, with a spatial resolution of up to ~170 nm.
This research not only made significant progress in material design, but also promoted the development of imaging technology. The team from Nanjing University successfully enhanced the electrochemical luminescence (ECL) performance of gold nanoclusters by precisely regulating the interactions between ligands. They rigidified the structure of gold nanoclusters using the hydrogen bond network within molecules, significantly increasing their luminescence efficiency. They then constructed an efficient luminescent interface with a thickness approaching that of a single cluster using this ultra-bright probe. This "near-zero thickness ECL imaging" method solved the problems of insufficient brightness and inaccurate positioning of traditional ECL probes, enabling high-resolution imaging of subcellular structures. In the future, combined with super-resolution imaging techniques (such as stimulated emission depletion microscopy or single-molecule localization strategies), this near-zero thickness ECL platform is expected to further break through the optical diffraction limit and achieve electrochemical luminescence imaging with nanoscale resolution in the absence of external excitation light and zero background interference, providing a new tool for in situ visualization of dynamic interactions of subcellular organelles and membrane interface processes.
This achievement was recently published in Angewandte Chemie International Edition. The first author of the article is Zhou Kai, a doctoral student from Nanjing University. Professor Zhu Junjie and Associate Professor Chen Zixuan are the co-corresponding authors of this paper. The research was funded by projects from the National Natural Science Foundation of China, etc.







