已传文件:photo/1766544328.png
In the fields of solar energy conversion, photocatalysis and display technology, colloidal quantum dots (QDs) have been widely studied due to their tunable band structure and excellent optical properties. Unlike traditional molecular systems, a single quantum dot can simultaneously absorb multiple photons to form a multi-excited state. However, the main decay pathway of the multi-excited state is non-radiative Auger recombination, and the lifetime of the double-excited state is usually only a few hundred picoseconds, which sharply decreases with the increase in the number of excitons, severely limiting the effective utilization of multi-excited energy.
Recently, a research team led by Professor Tang Benzhong from the University of Science and Technology of Hong Kong and the University of Chinese Academy of Sciences (Shenzhen) and Researcher Wu Kaifeng from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, published their latest research results in the Journal of the American Chemical Society. In this study, the team innovatively constructed an energy acceptor system based on aggregation-induced emission molecules (AIEgens, TBBA), and fixed this molecule onto the surface of cadmium-free and lead-free ZnSe/ZnS core-shell quantum dots through covalent anchoring. This system achieved efficient conversion of quantum dot multi-excited energy to the triplet state of the AIE molecule through two-step efficient charge transfer. Ultrafast transient absorption spectroscopy results showed that even at an average exciton number of up to 5.2, the first step electron transfer efficiency could still reach 96%. Different from traditional acceptor molecules, the second step hole transfer process directly leads to the generation of the triplet state of the TBBA molecule, and the triplet yield is linearly related to the average exciton number, indicating that the triplet yields of the multi-excited state and the single-excited state are basically the same.
Based on this new mechanism, the research team successfully achieved the effective storage of quantum multi-exciton state energy in the molecular triplet state, significantly extending the multi-exciton lifetime from several tens of picoseconds to 200 microseconds. Furthermore, it was successfully applied to photochemical reactions. This not only significantly prolonged the survival time of multi-exciton energy but also provided a new material platform for achieving efficient conversion of light energy to chemical energy. More importantly, this study first expanded the application of AIE molecules to the field of charge/energy acceptors, demonstrating its unique potential in molecular photophysics and energy conversion research. Due to the use of environmentally friendly quantum dots without heavy metals (Cd, Pb) and the excellent energy capture characteristics of AIE molecules, this system is expected to play an important role in the fields of photocatalysis and photoelectronic devices in the future.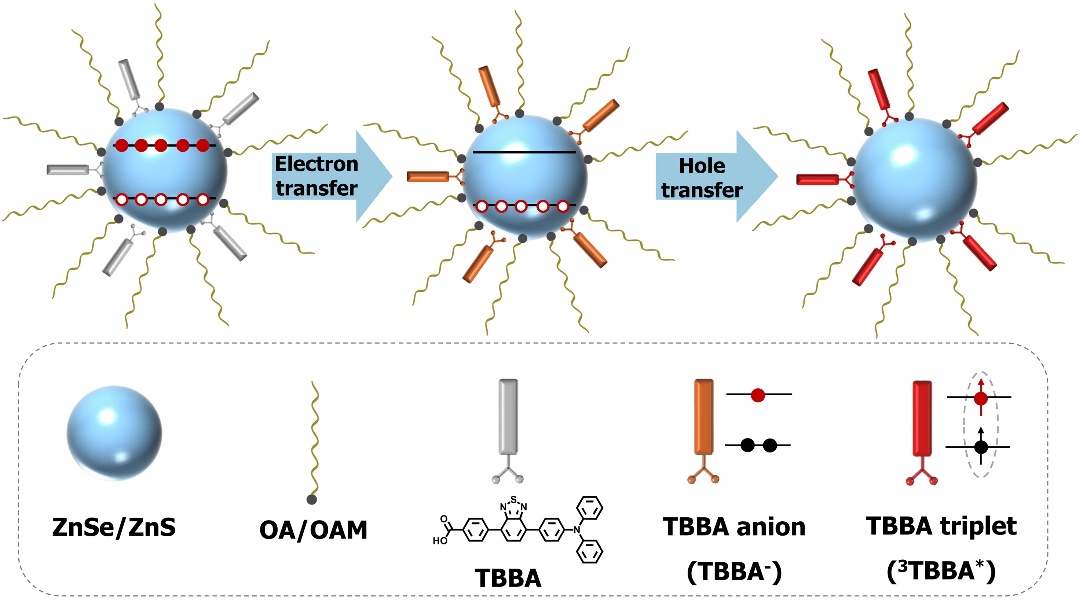
Figure 1. Multi-exciton energy transfer from ZnSe/ZnS quantum dots to TBBA molecules
The co-first authors of this study are Dr. He Shan from the Hong Kong University of Science and Technology and Dr. Xie Huilin. The corresponding author is Professor Wu Kaifeng from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Professor Tang Benzhong/Professor Zhang Jiancheng from the University of Chinese Academy of Sciences (Shenzhen), and Professor Lin Rongye from the Hong Kong University of Science and Technology. This study was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Basic Research Funds for Central Universities, the New Foundation Science Foundation, the Guangdong Provincial University Center for Basic Research on Colloids, the Shenzhen Peacock Team, the Hong Kong Research Grants Council, and the Innovation Technology Commission.




