
【Research Background】
Currently, it is a current trend to find effective, safe and low-cost energy storage systems to meet the growing demand for energy. Compared with other rechargeable batteries, sodium ion batteries (SIBs) have the advantages of high energy density, low cost, and abundant natural sodium source, and have broad application prospects. However, sodium ion batteries have poor charge transfer and embedding power because the ionic radius of Na+ (1.02Å) is greater than the ionic radius of Li+ (0.76Å) and the volume change is more severe during reversible ion embedding/embedding. learn. Recently, the transition metal carbide MXene is more competitive with other two-dimensional materials due to its high electrical conductivity and specific capacity.
[Introduction]
Recently, Professor Chengxiang Wang of Shandong University and Prof. Longwei Yin reported a novel strategy to couple NiCoP bimetallic phosphide nanoparticles with alkali-induced 3D interconnected pleated porous Ti3C2 MXene as the negative electrode of high performance sodium ion battery. Structural stability and improved reaction kinetics. The interconnected three-dimensional Ti3C2 pleat structure can create a rich pore size and a large specific surface area of 3D conductive space to promote charge and electrolyte ion transfer. The unique MXene structure effectively limits volume expansion and prevents aggregation of NiCoP nanoparticles during Na+ insertion/embedding. NiCoP has abundant redox reaction active sites, high conductivity and low charge transfer resistance. The synergistic effect of NiCoP and MXene Ti3C2 with structural stability and electrochemical activity makes it have excellent electrochemical performance, and the specific capacity of 261.7 mAh g -1 can be maintained after 2000 cycles at a current density of 1 A/g.
The results are published online in Energy & Environmental Science: Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries
[Graphic introduction]
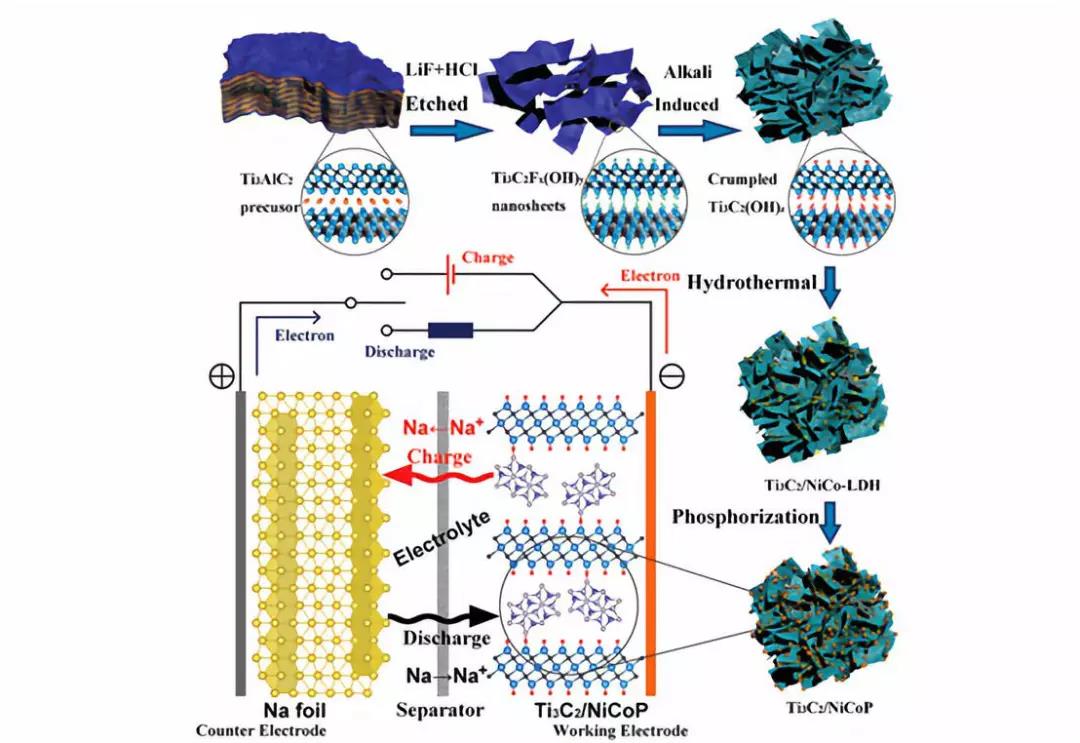
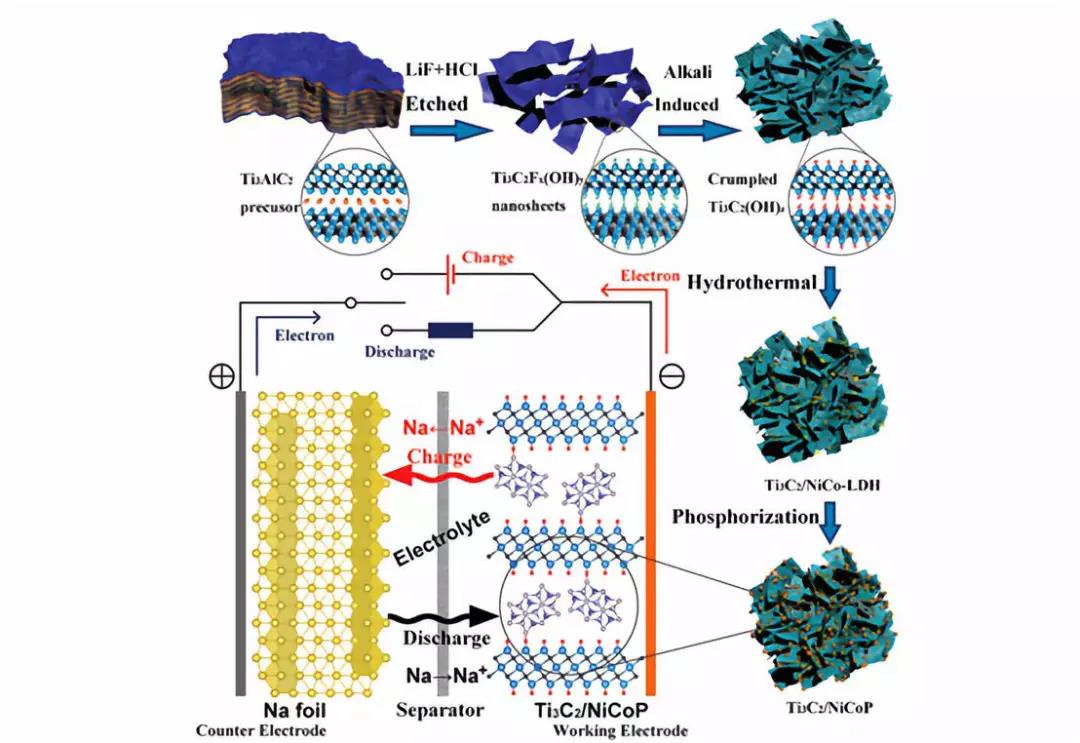
Schematic diagram of the synthesis process of Ti3C2/NiCoP hybrids and the mechanism of the half-cell.
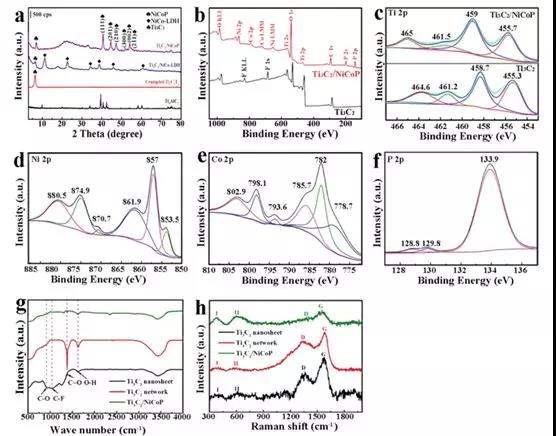
Figure 1 (a) XRD patterns of different samples, Ti3AlC2 precursor (black), alkali-induced fold Ti3C2 framework (red), Ti3C2 / NiCo-LDH (blue) and Ti3C2/NiCoP (purple); (b) Phosphating XPS spectra of Ti 2p before and after and (c) high resolution XPS spectra (df) Ni 2p of Ti3C2 / NiCoP hybrids, high resolution XPS spectra of Co 2p and P 2p (g) Ti3C2 nanosheets (black), Raman spectra of alkali-induced 3D Ti3C2 network (red) and Ti3C2/NiCoP hybrid (green)
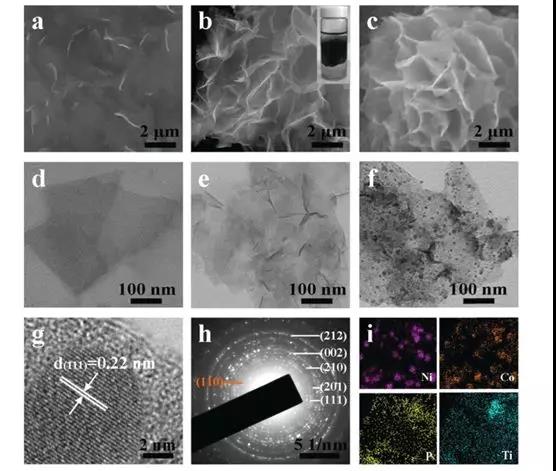
Figure 2 (a) Ti3C2 nanosheets; (b) alkali-induced 3D pleated Ti3C2 network and (c) FESEM image of Ti3C2 / NiCoP hybrid; (df) Ti3C2 nanosheets, alkali-induced 3D Ti3C2 network and Ti3C2 / NiCoP Low-power TEM image of the compound (g) HRTEM lattice image shows that the Ni-P (111) plane corresponds to a d-spacing of 0.22 nm (h) Electron diffraction pattern of Ti3C2 / NiCoP hybrid (i) EDX element of Ti3C2 / NiCoP hybrid Map showing the uniform distribution of Ni, Co, P and Ti elements in the hybrid
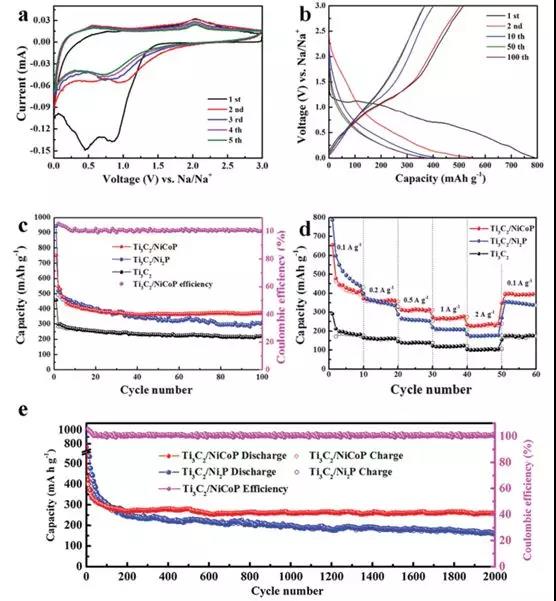
Figure 3 (a) CV curve of Ti3C2/NiCoP electrode and (b) discharge curve; (c) Cyclic performance and (d) rate performance of Ti3C2, Ti3C2/Ni2P and Ti3C2/NiCoP electrodes (e) Ti3C2 after 2000 cycles Long Cycle Performance of Ni/P and Ti3C2 / NiCoP Electrodes at Current Density of 1.0 Ag-1
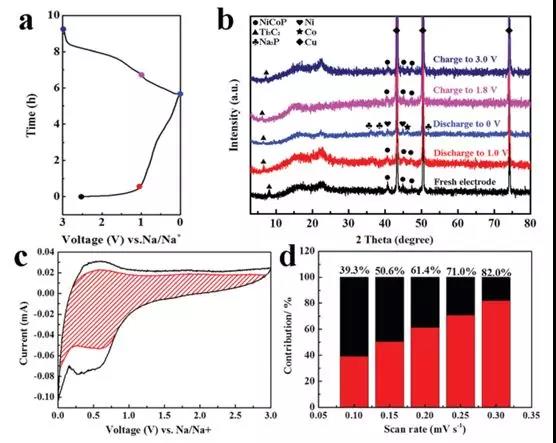
Figure 4 (a) First discharge-charge profile of the Ti3C2 / NiCoP electrode; (b) Ex-situ XRD pattern of the Ti3C2/NiCoP electrode at different discharge-charge phases; (c) Capacitance contribution (red region) And the diffusion contribution at 0.3 mV s-1 (d) at different scan rates of 0.1, 0.15, 0.2, 0.25 and 0.3 mV s-1.
[Summary of this article]
The unique 3D interconnected porous pleated Ti3C2/NiCoP hybrids were synthesized by solvothermal reaction and in-situ phosphating process. NiCoP nanoparticles were uniformly grown on Ti3C2 nanosheets to reduce volume change and improve the reaction kinetics of sodium ion batteries. . The synergistic effect between the components makes the NiCoP / Ti3C2 electrode have higher structural stability and electrochemical activity, thereby promoting electrochemical energy storage capacity. Ti3C2 establishes a 3D conductive network that not only limits the large volume expansion, but also prevents the aggregation of NiCoP nanoparticles during the insertion/embedding process of Na+, providing a 3D conductive space for the fast charge transfer process. At the same time, by introducing a binary metal to form a bimetallic phosphide, a synergistic effect is produced and the electrochemical performance is improved. The strategy of coupling phosphide to 3D Ti3C2 by in-situ phosphating can be extended to other designs for electrode materials for high performance energy storage devices.
Literature link:
DOI: 10.1039/c9ee00308h
Source: WeChat public account MXene Frontier










